Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease
- PMID: 23019375
- PMCID: PMC3465410
- DOI: 10.1073/pnas.1205102109
Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease
Erratum in
- Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11212
Abstract
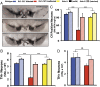
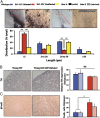
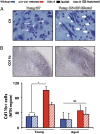
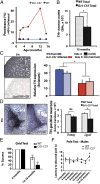
DJ-1 mutations cause autosomal recessive early-onset Parkinson disease (PD). We report a model of PD pathology: the DJ1-C57 mouse. A subset of DJ-1-nullizygous mice, when fully backcrossed to a C57BL/6 [corrected] background, display dramatic early-onset unilateral loss of dopaminergic (DA) neurons in their substantia nigra pars compacta, progressing to bilateral degeneration of the nigrostriatal axis with aging. In addition, these mice exhibit age-dependent bilateral degeneration at the locus ceruleus nucleus and display mild motor behavior deficits at aged time points. These findings effectively recapitulate the early stages of PD. Therefore, the DJ1-C57 mouse provides a tool to study the preclinical aspects of neurodegeneration. Importantly, by exome sequencing, we identify candidate modifying genes that segregate with the phenotype, providing potentially critical clues into how certain genes may influence the penetrance of DJ-1-related degeneration in mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Complex network-driven view of genomic mechanisms underlying Parkinson's disease: analyses in dorsal motor vagal nucleus, locus coeruleus, and substantia nigra.Biomed Res Int. 2014;2014:543673. doi: 10.1155/2014/543673. Epub 2014 Nov 26. Biomed Res Int. 2014. PMID: 25525598 Free PMC article.
-
Accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms.Brain. 2013 Aug;136(Pt 8):2369-78. doi: 10.1093/brain/awt196. Brain. 2013. PMID: 23884809
-
Repulsive Guidance Molecule a (RGMa) Induces Neuropathological and Behavioral Changes That Closely Resemble Parkinson's Disease.J Neurosci. 2017 Sep 27;37(39):9361-9379. doi: 10.1523/JNEUROSCI.0084-17.2017. Epub 2017 Aug 21. J Neurosci. 2017. PMID: 28842419 Free PMC article.
-
Molecular mechanisms of selective dopaminergic neuronal death in Parkinson's disease.Trends Mol Med. 2003 Mar;9(3):126-32. doi: 10.1016/s1471-4914(03)00020-0. Trends Mol Med. 2003. PMID: 12657434 Review.
-
Locus coeruleus.Cell Tissue Res. 2018 Jul;373(1):221-232. doi: 10.1007/s00441-017-2649-1. Epub 2017 Jul 7. Cell Tissue Res. 2018. PMID: 28687925 Review.
Cited by
-
Human Induced Pluripotent Stem Cell Phenotyping and Preclinical Modeling of Familial Parkinson's Disease.Genes (Basel). 2022 Oct 25;13(11):1937. doi: 10.3390/genes13111937. Genes (Basel). 2022. PMID: 36360174 Free PMC article. Review.
-
Surprising behavioral and neurochemical enhancements in mice with combined mutations linked to Parkinson's disease.Neurobiol Dis. 2014 Feb;62:113-23. doi: 10.1016/j.nbd.2013.09.009. Epub 2013 Sep 26. Neurobiol Dis. 2014. PMID: 24075852 Free PMC article.
-
Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans.Environ Toxicol Pharmacol. 2018 Jan;57:46-52. doi: 10.1016/j.etap.2017.11.005. Epub 2017 Nov 20. Environ Toxicol Pharmacol. 2018. PMID: 29190595 Free PMC article.
-
The interfaces between signal transduction pathways: IGF-1/mTor, p53 and the Parkinson Disease pathway.Oncotarget. 2012 Nov;3(11):1301-7. doi: 10.18632/oncotarget.759. Oncotarget. 2012. PMID: 23211569 Free PMC article. No abstract available.
-
Parkinson's disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2111338119. doi: 10.1073/pnas.2111338119. Proc Natl Acad Sci U S A. 2022. PMID: 35046029 Free PMC article.
References
-
- Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Hague S, et al. Early-onset Parkinson’s disease caused by a compound heterozygous DJ-1 mutation. Ann Neurol. 2003;54:271–274. - PubMed
-
- Kitada T, Tomlinson JJ, Ao HS, Grimes DA, Schlossmacher MG. Considerations regarding the etiology and future treatment of autosomal recessive versus idiopathic Parkinson disease. Curr Treat Options Neurol. 2012;14:230–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

