Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial
- PMID: 23015655
- PMCID: PMC3513535
- DOI: 10.1210/jc.2012-2794
Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial
Abstract
Context: Obesity is associated with reduced GH secretion and increased cardiovascular disease risk.
Objective: We performed this study to determine the effects of augmenting endogenous GH secretion on body composition and cardiovascular disease risk indices in obese subjects with reduced GH secretion.
Design, patients and methods: A randomized, double-blind, placebo-controlled study was performed involving 60 abdominally obese subjects with reduced GH secretion. Subjects received tesamorelin, a GHRH(1-44) analog, 2 mg once daily, or placebo for 12 months. Abdominal visceral adipose tissue (VAT) was assessed by abdominal computed tomography scan, and carotid intima-media thickness (cIMT) was assessed by ultrasound. Treatment effect was determined by longitudinal linear mixed-effects modeling.
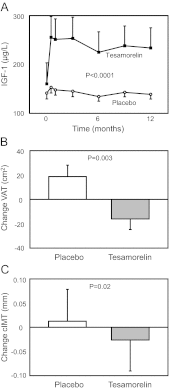
Results: VAT [-16 ± 9 vs.19 ± 9 cm(2), tesamorelin vs. placebo; treatment effect (95% confidence interval): -35 (-58, -12) cm(2); P = 0.003], cIMT (-0.03 ± 0.01 vs. 0.01 ± 0.01 mm; -0.04 (-0.07, -0.01) mm; P = 0.02), log C-reactive protein (-0.17 ± 0.04 vs. -0.03 ± 0.05 mg/liter; -0.15 (-0.30, -0.01) mg/liter, P = 0.04), and triglycerides (-26 ± 16 vs. 12 ± 8 mg/dl; -37 (-67, -7) mg/dl; P = 0.02) improved significantly in the tesamorelin group vs. placebo. No significant effects on abdominal sc adipose tissue (-6 ± 6 vs. 3 ± 11 cm(2); -10 (-32, +13) cm(2); P = 0.40) were seen. IGF-I increased (86 ± 21 vs. -6 ± 8 μg/liter; 92 (+52, +132) μg/liter; P < 0.0001). No changes in fasting, 2-h glucose, or glycated hemoglobin were seen. There were no serious adverse events or differences in adverse events between the groups.
Conclusion: Among obese subjects with relative reductions in GH, tesamorelin selectively reduces VAT without significant effects on sc adipose tissue and improves triglycerides, C-reactive protein, and cIMT, without aggravating glucose.
Trial registration: ClinicalTrials.gov NCT00675506.
Figures
Similar articles
-
The effects of tesamorelin on phosphocreatine recovery in obese subjects with reduced GH.J Clin Endocrinol Metab. 2014 Jan;99(1):338-43. doi: 10.1210/jc.2013-3436. Epub 2013 Dec 20. J Clin Endocrinol Metab. 2014. PMID: 24178787 Free PMC article. Clinical Trial.
-
Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity.J Clin Endocrinol Metab. 2013 Sep;98(9):3864-72. doi: 10.1210/jc.2013-2063. Epub 2013 Jul 3. J Clin Endocrinol Metab. 2013. PMID: 23824419 Free PMC article. Clinical Trial.
-
Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension.J Acquir Immune Defic Syndr. 2010 Mar;53(3):311-22. doi: 10.1097/QAI.0b013e3181cbdaff. J Acquir Immune Defic Syndr. 2010. PMID: 20101189 Clinical Trial.
-
Growth hormone axis treatments for HIV-associated lipodystrophy: a systematic review of placebo-controlled trials.HIV Med. 2011 Sep;12(8):453-62. doi: 10.1111/j.1468-1293.2010.00906.x. Epub 2011 Jan 25. HIV Med. 2011. PMID: 21265979 Review.
-
Effects of growth hormone-releasing hormone on visceral fat, metabolic, and cardiovascular indices in human studies.Growth Horm IGF Res. 2015 Apr;25(2):59-65. doi: 10.1016/j.ghir.2014.12.005. Epub 2014 Dec 20. Growth Horm IGF Res. 2015. PMID: 25555516 Free PMC article. Review.
Cited by
-
Growth hormone-releasing hormone is produced by adipocytes and regulates lipolysis through growth hormone receptor.Int J Obes (Lond). 2017 Oct;41(10):1547-1555. doi: 10.1038/ijo.2017.145. Epub 2017 Jun 19. Int J Obes (Lond). 2017. PMID: 28626214
-
The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations.Nat Rev Endocrinol. 2013 Jun;9(6):346-56. doi: 10.1038/nrendo.2013.64. Epub 2013 Apr 9. Nat Rev Endocrinol. 2013. PMID: 23568441 Review.
-
The effects of tesamorelin on phosphocreatine recovery in obese subjects with reduced GH.J Clin Endocrinol Metab. 2014 Jan;99(1):338-43. doi: 10.1210/jc.2013-3436. Epub 2013 Dec 20. J Clin Endocrinol Metab. 2014. PMID: 24178787 Free PMC article. Clinical Trial.
-
Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity.J Clin Endocrinol Metab. 2013 Sep;98(9):3864-72. doi: 10.1210/jc.2013-2063. Epub 2013 Jul 3. J Clin Endocrinol Metab. 2013. PMID: 23824419 Free PMC article. Clinical Trial.
-
Treatment with Growth Hormone for Adults with Growth Hormone Deficiency Syndrome: Benefits and Risks.Int J Mol Sci. 2018 Mar 17;19(3):893. doi: 10.3390/ijms19030893. Int J Mol Sci. 2018. PMID: 29562611 Free PMC article. Review.
References
-
- Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. 1990. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10:497–511 - PubMed
-
- Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. 2003. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 284:E1065–E1071 - PubMed
-
- Lemieux I, Pascot A, Prud'homme D, Alméras N, Bogaty P, Nadeau A, Bergeron J, Després JP. 2001. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 21:961–967 - PubMed
-
- Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB. 2007. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke 38:2422–2429 - PubMed
-
- Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. 1999. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care 22:1808–1812 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K24DK064545/DK/NIDDK NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- UL1RR025758/RR/NCRR NIH HHS/United States
- M01RR01066/RR/NCRR NIH HHS/United States
- P30DK040561/DK/NIDDK NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- K23DK087857/DK/NIDDK NIH HHS/United States
- R01HL085268/HL/NHLBI NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- R01 HL085268/HL/NHLBI NIH HHS/United States
- K24 DK064545/DK/NIDDK NIH HHS/United States
- K23 DK087857/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

