Clathrin is not required for SNX-BAR-retromer-mediated carrier formation
- PMID: 23015596
- PMCID: PMC3603510
- DOI: 10.1242/jcs.112904
Clathrin is not required for SNX-BAR-retromer-mediated carrier formation
Abstract
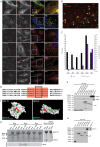
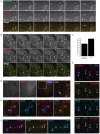
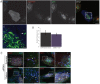
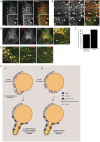
Clathrin has been implicated in retromer-mediated trafficking, but its precise function remains elusive. Given the importance of retromers for efficient endosomal sorting, we have sought to clarify the relationship between clathrin and the SNX-BAR retromer. We find that the retromer SNX-BARs do not interact directly or indirectly with clathrin. In addition, we observe that SNX-BAR-retromer tubules and carriers are not clathrin coated. Furthermore, perturbing clathrin function, by overexpressing a dominant-negative clathrin or through suppression of clathrin expression, has no detectable effect on the frequency of SNX-BAR-retromer tubulation. We propose that SNX-BAR-retromer-mediated membrane deformation and carrier formation does not require clathrin, and hence the role of clathrin in SNX-BAR-retromer function would appear to lie in pre-SNX-BAR-retromer cargo sorting.
Figures
Similar articles
-
SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation.Traffic. 2012 Jan;13(1):94-107. doi: 10.1111/j.1600-0854.2011.01297.x. Epub 2011 Oct 31. Traffic. 2012. PMID: 21973056
-
SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting.Semin Cell Dev Biol. 2010 Jun;21(4):371-80. doi: 10.1016/j.semcdb.2009.11.009. Epub 2009 Nov 13. Semin Cell Dev Biol. 2010. PMID: 19914387 Free PMC article. Review.
-
Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport.J Cell Biol. 2017 Nov 6;216(11):3677-3693. doi: 10.1083/jcb.201702137. Epub 2017 Sep 21. J Cell Biol. 2017. PMID: 28935632 Free PMC article.
-
Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8.EMBO J. 2009 Nov 4;28(21):3290-302. doi: 10.1038/emboj.2009.272. Epub 2009 Sep 17. EMBO J. 2009. PMID: 19763082 Free PMC article.
-
You can go your own way: SNX-BAR coat complexes direct traffic at late endosomes.Curr Opin Cell Biol. 2022 Jun;76:102087. doi: 10.1016/j.ceb.2022.102087. Epub 2022 May 12. Curr Opin Cell Biol. 2022. PMID: 35569261 Review.
Cited by
-
Chemical-genetic disruption of clathrin function spares adaptor complex 3-dependent endosome vesicle biogenesis.Mol Biol Cell. 2013 Aug;24(15):2378-88. doi: 10.1091/mbc.E12-12-0860. Epub 2013 Jun 12. Mol Biol Cell. 2013. PMID: 23761069 Free PMC article.
-
Receptor-mediated Endocytosis 8 Utilizes an N-terminal Phosphoinositide-binding Motif to Regulate Endosomal Clathrin Dynamics.J Biol Chem. 2015 Aug 28;290(35):21676-89. doi: 10.1074/jbc.M115.644757. Epub 2015 Jul 1. J Biol Chem. 2015. PMID: 26134565 Free PMC article.
-
Dynamics and instabilities of lipid bilayer membrane shapes.Adv Colloid Interface Sci. 2014 Jun;208:76-88. doi: 10.1016/j.cis.2014.01.004. Epub 2014 Jan 25. Adv Colloid Interface Sci. 2014. PMID: 24529968 Free PMC article. Review.
-
Endosomal microdomains: Formation and function.Curr Opin Cell Biol. 2020 Aug;65:86-95. doi: 10.1016/j.ceb.2020.02.018. Epub 2020 Apr 1. Curr Opin Cell Biol. 2020. PMID: 32247230 Free PMC article. Review.
-
Assembly and fission of tubular carriers mediating protein sorting in endosomes.Nat Rev Mol Cell Biol. 2024 Oct;25(10):765-783. doi: 10.1038/s41580-024-00746-8. Epub 2024 Jun 17. Nat Rev Mol Cell Biol. 2024. PMID: 38886588 Review.
References
-
- Carlton J., Bujny M., Peter B. J., Oorschot V. M., Rutherford A., Mellor H., Klumperman J., McMahon H. T., Cullen P. J. (2004). Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr. Biol. 14, 1791–1800 10.1016/j.cub.2004.09.077 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

