DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells
- PMID: 23012257
- PMCID: PMC3533478
- DOI: 10.1124/mol.112.080457
DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells
Abstract
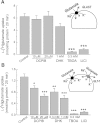
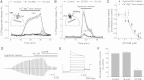
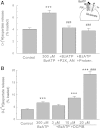
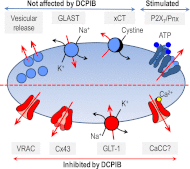
4-(2-Butyl-6,7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxobutyric acid (DCPIB) was identified as the selective blocker of volume-regulated anion channels (VRAC). VRAC are permeable to small inorganic and organic anions, including the excitatory neurotransmitter glutamate. In recent years DCPIB has been increasingly used for probing the physiologic and pathologic roles of VRAC and was found to potently suppress pathologic glutamate release in cerebral ischemia. Because ischemic glutamate release can be mediated by a plethora of mechanisms, in this study we explored the selectivity of DCPIB toward the majority of previously identified glutamate transporters and permeability pathways. l-[(3)H]glutamate, d-[(3)H]aspartate, and l-[(14)C]cystine were used to trace amino acid release and uptake. We found that in addition to its well-characterized effect on VRAC, DCPIB potently inhibited glutamate release via connexin hemichannels and glutamate uptake via the glutamate transporter GLT-1 in rat glial cells. In contrast, DCPIB had no direct effect on vesicular glutamate release from rat brain synaptosomes or the cystine/glutamate exchange in astrocytes. The compound did not affect the astrocytic glutamate transporter GLAST, nor did it block glutamate release via the P2X(7)/pannexin permeability pathway. The ability of DCPIB to directly block connexin hemichannels was confirmed using a gene-specific siRNA knockdown approach. Overall, our data demonstrate that DCPIB influences several glutamate transport pathways and that its effects on VRAC in vivo should be verified using additional pharmacological controls.
Figures




Similar articles
-
DCPIB, a potent volume-regulated anion channel antagonist, attenuates microglia-mediated inflammatory response and neuronal injury following focal cerebral ischemia.Brain Res. 2014 Jan 13;1542:176-85. doi: 10.1016/j.brainres.2013.10.026. Epub 2013 Nov 1. Brain Res. 2014. PMID: 24189520
-
DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra.Exp Neurol. 2008 Apr;210(2):514-20. doi: 10.1016/j.expneurol.2007.11.027. Epub 2007 Dec 7. Exp Neurol. 2008. PMID: 18206872 Free PMC article.
-
DCPIB, a specific inhibitor of volume-regulated anion channels (VRACs), inhibits astrocyte proliferation and cell cycle progression via G1/S arrest.J Mol Neurosci. 2012 Feb;46(2):249-57. doi: 10.1007/s12031-011-9524-4. Epub 2011 May 11. J Mol Neurosci. 2012. PMID: 21559876
-
Mechanisms of glutamate release from astrocytes.Neurochem Int. 2008 Jan;52(1-2):142-54. doi: 10.1016/j.neuint.2007.06.005. Epub 2007 Jun 26. Neurochem Int. 2008. PMID: 17669556 Free PMC article. Review.
-
SLC1 glutamate transporters.Pflugers Arch. 2014 Jan;466(1):3-24. doi: 10.1007/s00424-013-1397-7. Epub 2013 Nov 19. Pflugers Arch. 2014. PMID: 24240778 Free PMC article. Review.
Cited by
-
LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion.Nat Commun. 2018 May 17;9(1):1974. doi: 10.1038/s41467-018-04353-y. Nat Commun. 2018. PMID: 29773801 Free PMC article.
-
Osmotic Edema Rapidly Increases Neuronal Excitability Through Activation of NMDA Receptor-Dependent Slow Inward Currents in Juvenile and Adult Hippocampus.ASN Neuro. 2015 Oct 21;7(5):1759091415605115. doi: 10.1177/1759091415605115. Print 2015 Sep-Oct. ASN Neuro. 2015. PMID: 26489684 Free PMC article.
-
Conditional deletion of LRRC8A in the brain reduces stroke damage independently of swelling-activated glutamate release.iScience. 2023 Apr 14;26(5):106669. doi: 10.1016/j.isci.2023.106669. eCollection 2023 May 19. iScience. 2023. PMID: 37182109 Free PMC article.
-
Hypoxia, Ion Channels and Glioblastoma Malignancy.Biomolecules. 2023 Dec 4;13(12):1742. doi: 10.3390/biom13121742. Biomolecules. 2023. PMID: 38136613 Free PMC article. Review.
-
Inhibition of the LRRC8A channel promotes microglia/macrophage phagocytosis and improves outcomes after intracerebral hemorrhagic stroke.iScience. 2022 Nov 13;25(12):105527. doi: 10.1016/j.isci.2022.105527. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465125 Free PMC article.
References
-
- Akita T, Fedorovich SV, Okada Y. (2011) Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28:1181–1190 - PubMed
-
- Bannai S. (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 261:2256–2263 - PubMed
-
- Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, Nobile M, Ferroni S. (2009) Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 3:323–336 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
