Jak-STAT regulation of cyst stem cell development in the Drosophila testis
- PMID: 23010510
- PMCID: PMC3481170
- DOI: 10.1016/j.ydbio.2012.09.009
Jak-STAT regulation of cyst stem cell development in the Drosophila testis
Abstract
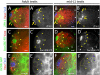
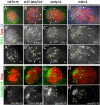
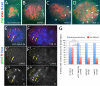
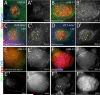
Establishment and maintenance of functional stem cells is critical for organ development and tissue homeostasis. Little is known about the mechanisms underlying stem establishment during organogenesis. Drosophila testes are among the most thoroughly characterized systems for studying stem cell behavior, with germline stem cells (GSCs) and somatic cyst stem cells (CySCs) cohabiting a discrete stem cell niche at the testis apex. GSCs and CySCs are arrayed around hub cells that also comprise the niche and communication between hub cells, GSCs, and CySCs regulates the balance between stem cell maintenance and differentiation. Recent data has shown that functional, asymmetrically dividing GSCs are first established at ∼23 h after egg laying during Drosophila testis morphogenesis (Sheng et al., 2009). This process correlates with coalescence of the hub, but development of CySCs from somatic gonadal precursors (SGPs) was not examined. Here, we show that functional CySCs are present at the time of GSC establishment, and that Jak-STAT signaling is necessary and sufficient for CySC maintenance shortly thereafter. Furthermore, hyper-activation of Jak in CySCs promotes expansion of the GSC population, while ectopic Jak activation in the germline induces GSC gene expression in GSC daughter cells but does not prevent spermatogenic differentiation. Together, these observations indicate that, similar to adult testes, Jak-STAT signaling from the hub acts on both GSCs and CySC to regulate their development and differentiation, and that additional signaling from CySCs to the GSCs play a dominant role in controlling GSC maintenance during niche formation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling.J Cell Physiol. 2010 May;223(2):500-10. doi: 10.1002/jcp.22073. J Cell Physiol. 2010. PMID: 20143337 Free PMC article.
-
A regulatory loop of JAK/STAT signalling and its downstream targets represses cell fate conversion and maintains male germline stem cell niche homeostasis.Cell Prolif. 2024 Oct;57(10):e13648. doi: 10.1111/cpr.13648. Epub 2024 Jul 10. Cell Prolif. 2024. PMID: 38987866 Free PMC article.
-
The Drosophila BCL6 homolog Ken and Barbie promotes somatic stem cell self-renewal in the testis niche.Dev Biol. 2012 Aug 15;368(2):181-92. doi: 10.1016/j.ydbio.2012.04.034. Epub 2012 May 8. Dev Biol. 2012. PMID: 22580161 Free PMC article.
-
Hedgehog in the Drosophila testis niche: what does it do there?Protein Cell. 2013 Sep;4(9):650-5. doi: 10.1007/s13238-013-3040-y. Epub 2013 Jun 26. Protein Cell. 2013. PMID: 23807635 Free PMC article. Review.
-
Local signaling within stem cell niches: insights from Drosophila.Curr Opin Cell Biol. 2012 Apr;24(2):225-31. doi: 10.1016/j.ceb.2012.01.004. Epub 2012 Jan 30. Curr Opin Cell Biol. 2012. PMID: 22296770 Free PMC article. Review.
Cited by
-
Enhancer of polycomb coordinates multiple signaling pathways to promote both cyst and germline stem cell differentiation in the Drosophila adult testis.PLoS Genet. 2017 Feb 14;13(2):e1006571. doi: 10.1371/journal.pgen.1006571. eCollection 2017 Feb. PLoS Genet. 2017. PMID: 28196077 Free PMC article.
-
A conserved function of Human DLC3 and Drosophila Cv-c in testis development.Elife. 2022 Nov 3;11:e82343. doi: 10.7554/eLife.82343. Elife. 2022. PMID: 36326091 Free PMC article.
-
An actomyosin network organizes niche morphology and responds to feedback from recruited stem cells.bioRxiv [Preprint]. 2024 Aug 7:2023.09.08.556877. doi: 10.1101/2023.09.08.556877. bioRxiv. 2024. Update in: Curr Biol. 2024 Sep 9;34(17):3917-3930.e6. doi: 10.1016/j.cub.2024.07.041. PMID: 38746236 Free PMC article. Updated. Preprint.
-
An actomyosin network organizes niche morphology and responds to feedback from recruited stem cells.Curr Biol. 2024 Sep 9;34(17):3917-3930.e6. doi: 10.1016/j.cub.2024.07.041. Epub 2024 Aug 12. Curr Biol. 2024. PMID: 39137785
-
Sonication-facilitated immunofluorescence staining of late-stage embryonic and larval Drosophila tissues in situ.J Vis Exp. 2014 Aug 14;(90):e51528. doi: 10.3791/51528. J Vis Exp. 2014. PMID: 25146311 Free PMC article.
References
-
- Aboim AN. Developpement embryonnaire et post-embryonnaire des gonades normales et agametiques de Drosophila melanogaster. Revue Suisse de Zoologie. 1945;52:53–154.
-
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. - PubMed
-
- Baksa K, Parke T, Dobens LL, Dearolf CR. The Drosophila STAT protein, stat92E, regulates follicle cell differentiation during oogenesis. Developmental Biology. 2002;243:166–175. - PubMed
-
- Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124:971–982. - PubMed
-
- Boyle M, DiNardo S. Specification, migration, and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

