Virus-like particles as universal influenza vaccines
- PMID: 23002980
- PMCID: PMC3513402
- DOI: 10.1586/erv.12.70
Virus-like particles as universal influenza vaccines
Abstract
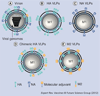
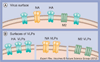
Current influenza vaccines are primarily targeted to induce immunity to the influenza virus strain-specific hemagglutinin antigen and are not effective in controlling outbreaks of new pandemic viruses. An approach for developing universal vaccines is to present highly conserved antigenic epitopes in an immunogenic conformation such as virus-like particles (VLPs) together with an adjuvant to enhance the vaccine immunogenicity. In this review, the authors focus on conserved antigenic targets and molecular adjuvants that were presented in VLPs. Conserved antigenic targets that include the hemagglutinin stalk domain, the external domain of influenza M2 and neuraminidase are discussed in addition to molecular adjuvants that are engineered to be incorporated into VLPs in a membrane-anchored form.
Figures

Similar articles
-
Highly immunogenic influenza virus-like particles containing B-cell-activating factor (BAFF) for multi-subtype vaccine development.Antiviral Res. 2019 Apr;164:12-22. doi: 10.1016/j.antiviral.2019.02.004. Epub 2019 Feb 6. Antiviral Res. 2019. PMID: 30738089
-
Incorporation of conserved nucleoprotein into influenza virus-like particles could provoke a broad protective immune response in BALB/c mice and chickens.Virus Res. 2015 Jan 2;195:35-42. doi: 10.1016/j.virusres.2014.09.018. Virus Res. 2015. PMID: 25312452
-
A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus.PLoS One. 2012;7(8):e42363. doi: 10.1371/journal.pone.0042363. Epub 2012 Aug 7. PLoS One. 2012. PMID: 22879951 Free PMC article.
-
Universal Influenza Vaccines: Progress in Achieving Broad Cross-Protection In Vivo.Am J Epidemiol. 2018 Dec 1;187(12):2603-2614. doi: 10.1093/aje/kwy145. Am J Epidemiol. 2018. PMID: 30084906 Free PMC article. Review.
-
Novel vaccines against influenza viruses.Virus Res. 2011 Dec;162(1-2):31-8. doi: 10.1016/j.virusres.2011.09.037. Epub 2011 Oct 1. Virus Res. 2011. PMID: 21968298 Free PMC article. Review.
Cited by
-
Novel vaccine strategies against emerging viruses.Curr Opin Virol. 2013 Apr;3(2):210-6. doi: 10.1016/j.coviro.2013.02.001. Epub 2013 Mar 7. Curr Opin Virol. 2013. PMID: 23477832 Free PMC article. Review.
-
CD47 Plays a Role as a Negative Regulator in Inducing Protective Immune Responses to Vaccination against Influenza Virus.J Virol. 2016 Jul 11;90(15):6746-6758. doi: 10.1128/JVI.00605-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27194758 Free PMC article.
-
Supplementation of H1N1pdm09 split vaccine with heterologous tandem repeat M2e5x virus-like particles confers improved cross-protection in ferrets.Vaccine. 2016 Jan 20;34(4):466-473. doi: 10.1016/j.vaccine.2015.12.023. Epub 2015 Dec 19. Vaccine. 2016. PMID: 26709639 Free PMC article.
-
Elicitation of Highly Pathogenic Avian Influenza H5N1 M2e and HA2-Specific Humoral and Cell-Mediated Immune Response in Chicken Following Immunization With Recombinant M2e-HA2 Fusion Protein.Front Vet Sci. 2021 Feb 5;7:571999. doi: 10.3389/fvets.2020.571999. eCollection 2020. Front Vet Sci. 2021. PMID: 33614753 Free PMC article.
-
Development and efficacy evaluation of remodeled canine parvovirus-like particles displaying major antigenic epitopes of a giant panda derived canine distemper virus.Front Microbiol. 2023 Feb 27;14:1117135. doi: 10.3389/fmicb.2023.1117135. eCollection 2023. Front Microbiol. 2023. PMID: 36922967 Free PMC article.
References
-
- Osterholm MT. Preparing for the next pandemic. N. Engl. J. Med. 2005;352(18):1839–1842. - PubMed
-
- Palese P, Compans RW. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J. Gen. Virol. 1976;33(1):159–163. - PubMed
-
- Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004;103(1–2):177–185. - PubMed
Websites
-
- Universal influenza vaccine tested successfully in humans. Science Daily. 2008 Jan 24; www.sciencedaily.com/releases/2008/01/080124185522.htm.
-
- VaxInnate. VaxInnate’s Universal Flu Vaccine Candidate Shown Safe and Immunogenic in Phase I Clinical Study. VaxInnate, NJ, USA: 2008. www.vaxinnate.com/pages/pressreleases/20070925_001.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
