μ-Opioid receptor desensitization: homologous or heterologous?
- PMID: 23002724
- PMCID: PMC3527680
- DOI: 10.1111/ejn.12003
μ-Opioid receptor desensitization: homologous or heterologous?
Abstract
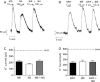
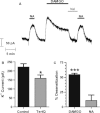
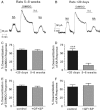
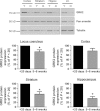
There is considerable controversy over whether μ-opioid receptor (MOPr) desensitization is homologous or heterologous and over the mechanisms underlying such desensitization. In different cell types MOPr desensitization has been reported to involve receptor phosphorylation by various kinases, including G-protein-coupled receptor kinases (GRKs), second messenger and other kinases as well as perturbation of the MOPr effector pathway by GRK sequestration of G protein βγ subunits or ion channel modulation. Here we report that in brainstem locus coeruleus (LC) neurons prepared from relatively mature rats (5-8 weeks old) rapid MOPr desensitization induced by the high-efficacy opioid peptides methionine enkephalin and DAMGO was homologous and not heterologous to α(2)-adrenoceptors and somatostatin SST(2) receptors. Given that these receptors all couple through G proteins to the same set of G-protein inwardly rectifying (GIRK) channels it is unlikely therefore that in mature neurons MOPr desensitization involves G protein βγ subunit sequestration or ion channel modulation. In contrast, in slices from immature animals (less than postnatal day 20), MOPr desensitization was observed to be heterologous and could be downstream of the receptor. Heterologous MOPr desensitization was not dependent on protein kinase C or c-Jun N-terminal kinase activity, but the change from heterologous to homologous desensitization with age was correlated with a decrease in the expression levels of GRK2 in the LC and other brain regions. The observation that the mechanisms underlying MOPr desensitization change with neuronal development is important when extrapolating to the mature brain results obtained from experiments on expression systems, cell lines and immature neuronal preparations.
© 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures

Similar articles
-
Role of G Protein-Coupled Receptor Kinases 2 and 3 in μ-Opioid Receptor Desensitization and Internalization.Mol Pharmacol. 2015 Aug;88(2):347-56. doi: 10.1124/mol.115.098293. Epub 2015 May 26. Mol Pharmacol. 2015. PMID: 26013542 Free PMC article.
-
Prolonged stimulation of μ-opioid receptors produces β-arrestin-2-mediated heterologous desensitization of α(2)-adrenoceptor function in locus ceruleus neurons.Mol Pharmacol. 2012 Sep;82(3):473-80. doi: 10.1124/mol.112.079350. Epub 2012 Jun 11. Mol Pharmacol. 2012. PMID: 22689562
-
G protein-coupled receptor kinase 2 mediates mu-opioid receptor desensitization in GABAergic neurons of the nucleus raphe magnus.J Neurochem. 2001 Apr;77(2):435-44. doi: 10.1046/j.1471-4159.2001.00267.x. J Neurochem. 2001. PMID: 11299306
-
Agonist-selective mechanisms of GPCR desensitization.Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S379-88. doi: 10.1038/sj.bjp.0707604. Epub 2007 Dec 3. Br J Pharmacol. 2008. PMID: 18059321 Free PMC article. Review.
-
Sex-dependent impact of microbiota status on cerebral μ-opioid receptor density in fischer rats.Eur J Neurosci. 2022 Apr;55(8):1917-1933. doi: 10.1111/ejn.15666. Epub 2022 Apr 24. Eur J Neurosci. 2022. PMID: 35393704 Free PMC article. Review.
Cited by
-
A bead-based GPCR phosphorylation immunoassay for high-throughput ligand profiling and GRK inhibitor screening.Commun Biol. 2022 Nov 9;5(1):1206. doi: 10.1038/s42003-022-04135-9. Commun Biol. 2022. PMID: 36352263 Free PMC article.
-
Ethanol reversal of cellular tolerance to morphine in rat locus coeruleus neurons.Mol Pharmacol. 2013 Aug;84(2):252-60. doi: 10.1124/mol.113.085936. Epub 2013 May 28. Mol Pharmacol. 2013. PMID: 23716621 Free PMC article.
-
Recent Progress in Opioid Research from an Electrophysiological Perspective.Mol Pharmacol. 2020 Oct;98(4):401-409. doi: 10.1124/mol.119.119040. Epub 2020 Mar 20. Mol Pharmacol. 2020. PMID: 32198208 Free PMC article. Review.
-
Cellular Tolerance Induced by Chronic Opioids in the Central Nervous System.Front Syst Neurosci. 2022 Jun 28;16:937126. doi: 10.3389/fnsys.2022.937126. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35837149 Free PMC article. Review.
-
Role of G Protein-Coupled Receptor Kinases 2 and 3 in μ-Opioid Receptor Desensitization and Internalization.Mol Pharmacol. 2015 Aug;88(2):347-56. doi: 10.1124/mol.115.098293. Epub 2015 May 26. Mol Pharmacol. 2015. PMID: 26013542 Free PMC article.
References
-
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitization of μ-opioid receptors in mature rat locus ceruleus neurons. Mol. Pharmacol. 2004;66:1592–1598. - PubMed
-
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in μ-opioid receptor desensitization and morphine tolerance? Trends Pharmacol. Sci. 2006;27:558–565. - PubMed
-
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, Henderson G. Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of μ-opioid receptors in mature brain neurons. Br. J. Pharmacol. 2009;158:157–164. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

