Cathepsin D acts as an essential mediator to promote malignancy of benign prostatic epithelium
- PMID: 22996917
- PMCID: PMC3594371
- DOI: 10.1002/pros.22589
Cathepsin D acts as an essential mediator to promote malignancy of benign prostatic epithelium
Abstract
Background: Stromal-epithelial interactions are important in both development and prostate cancer. Stromal changes have been shown to be powerful prognostic indicators of prostate cancer progression and of patient death helping to define lethal versus indolent phenotypes. The specific molecular underpinnings of these interactions are incompletely understood. We investigated whether stromal cathepsin D (CathD) overexpression affects prostate tumorigenesis through a paracrine mechanism.
Methods: Normal prostate fibroblasts (NPF) were retrovirally transduced to overexpress cyclin D1 (CD1) and were designated NPF(CD1) . Cathepsin D expression was knocked down using shRNA in cancer associated fibroblasts (CAF) and NPF(CD1) . We analyzed these stromal cell lines using immunohistochemistry, Western blot, and tissue recombination.
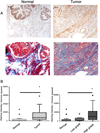
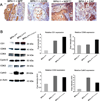
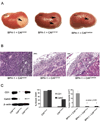
Results: An examination of human prostate tissue revealed significantly increased stromal staining of CathD in malignant prostate tissue. Overexpression of CD1 in normal prostate fibroblasts (NPF(CD1) ) produced a phenotype similar to, but more moderate than, CAF in a tissue recombination model. Knockdown studies revealed that CathD is required for NPF(CD1) motility and invasive growth in vitro. BPH-1 cell proliferation was found to be induced when cultured with NPF(CD1) conditioned medium, this effect was inhibited when CathD was knocked down in NPF(CD1) cells. Overexpression of CathD in prostate stromal cells induced malignancy in adjacent epithelium, and this transformation was inhibited when stromal CathD expression was knocked down in CAF.
Conclusions: The study presented here demonstrates increased CathD expression is seen in human CAF. The upregulation of CD1 results in concomitant increases in CathD expression. Elevated CathD expression in the stroma contributes to tumor promotion.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Active sonic hedgehog signaling between androgen independent human prostate cancer cells and normal/benign but not cancer-associated prostate stromal cells.Prostate. 2011 Dec;71(16):1711-22. doi: 10.1002/pros.21388. Epub 2011 Apr 25. Prostate. 2011. PMID: 21520153 Free PMC article.
-
Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast transdifferentiation.Mech Ageing Dev. 2005 Jan;126(1):59-69. doi: 10.1016/j.mad.2004.09.023. Mech Ageing Dev. 2005. PMID: 15610763
-
Tissue-specific consequences of cyclin D1 overexpression in prostate cancer progression.Cancer Res. 2007 Sep 1;67(17):8188-97. doi: 10.1158/0008-5472.CAN-07-0418. Cancer Res. 2007. PMID: 17804732
-
Role of the stromal microenvironment in carcinogenesis of the prostate.Int J Cancer. 2003 Oct 20;107(1):1-10. doi: 10.1002/ijc.11335. Int J Cancer. 2003. PMID: 12925950 Review.
-
Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model.Prostate. 1996 Feb;28(2):98-106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. Prostate. 1996. PMID: 8604398 Review.
Cited by
-
Therapeutic Potential of Marine Peptides in Prostate Cancer: Mechanistic Insights.Mar Drugs. 2022 Jul 22;20(8):466. doi: 10.3390/md20080466. Mar Drugs. 2022. PMID: 35892934 Free PMC article. Review.
-
Cathepsins mediate tumor metastasis.World J Biol Chem. 2013 Nov 26;4(4):91-101. doi: 10.4331/wjbc.v4.i4.91. World J Biol Chem. 2013. PMID: 24340132 Free PMC article. Review.
-
Protein signatures to distinguish aggressive from indolent prostate cancer.Prostate. 2022 Apr;82(5):605-616. doi: 10.1002/pros.24307. Epub 2022 Jan 31. Prostate. 2022. PMID: 35098564 Free PMC article.
-
Cellular senescence as a possible link between prostate diseases of the ageing male.Nat Rev Urol. 2021 Oct;18(10):597-610. doi: 10.1038/s41585-021-00496-8. Epub 2021 Jul 22. Nat Rev Urol. 2021. PMID: 34294916 Review.
-
The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery.Biomedicines. 2023 Dec 28;12(1):79. doi: 10.3390/biomedicines12010079. Biomedicines. 2023. PMID: 38255186 Free PMC article. Review.
References
-
- Weinberg RA. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494–495. - PubMed
-
- Li H, Fan X. Tumor microenvironment: the role of the tumor stroma in cancer. Journal of cellular biochemistry. 2007 - PubMed
-
- Cunha G. Tissue interactions between epithelium and mesenchyme of urogenital and integumental origin. The Anatomical Record. 1972 - PubMed
-
- Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

