TGFβ signalling in context
- PMID: 22992590
- PMCID: PMC4027049
- DOI: 10.1038/nrm3434
TGFβ signalling in context
Abstract
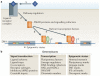
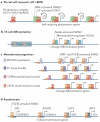
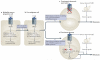
The basic elements of the transforming growth factor-β (TGFβ) pathway were revealed more than a decade ago. Since then, the concept of how the TGFβ signal travels from the membrane to the nucleus has been enriched with additional findings, and its multifunctional nature and medical relevance have relentlessly come to light. However, an old mystery has endured: how does the context determine the cellular response to TGFβ? Solving this question is key to understanding TGFβ biology and its many malfunctions. Recent progress is pointing at answers.
Figures




Similar articles
-
USP11 augments TGFβ signalling by deubiquitylating ALK5.Open Biol. 2012 Jun;2(6):120063. doi: 10.1098/rsob.120063. Open Biol. 2012. PMID: 22773947 Free PMC article.
-
Nitric oxide induces TIMP-1 expression by activating the transforming growth factor beta-Smad signaling pathway.J Biol Chem. 2005 Nov 25;280(47):39403-16. doi: 10.1074/jbc.M504140200. Epub 2005 Sep 23. J Biol Chem. 2005. PMID: 16183640
-
TGFβ receptor endocytosis and Smad signaling require synaptojanin1, PI3K-C2α-, and INPP4B-mediated phosphoinositide conversions.Mol Biol Cell. 2020 Mar 1;31(5):360-372. doi: 10.1091/mbc.E19-11-0662. Epub 2020 Jan 8. Mol Biol Cell. 2020. PMID: 31913757 Free PMC article.
-
Regulated intramembrane proteolysis of the TGFβ type I receptor conveys oncogenic signals.Future Oncol. 2014 Aug;10(11):1853-61. doi: 10.2217/fon.14.45. Epub 2014 Mar 5. Future Oncol. 2014. PMID: 24597658 Review.
-
Regulating the stability of TGFbeta receptors and Smads.Cell Res. 2009 Jan;19(1):21-35. doi: 10.1038/cr.2008.308. Cell Res. 2009. PMID: 19030025 Review.
Cited by
-
Doxorubicin restrains osteogenesis and promotes osteoclastogenesis in vitro.Am J Transl Res. 2020 Sep 15;12(9):5640-5654. eCollection 2020. Am J Transl Res. 2020. PMID: 33042445 Free PMC article.
-
Characterization of the roles of amphiregulin and transforming growth factor β1 in microvasculature-like formation in human granulosa-lutein cells.Front Cell Dev Biol. 2022 Aug 24;10:968166. doi: 10.3389/fcell.2022.968166. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092732 Free PMC article.
-
Bacterial Lipopolysaccharide Induces PD-L1 Expression and an Invasive Phenotype of Oral Squamous Cell Carcinoma Cells.Cancers (Basel). 2024 Jan 13;16(2):343. doi: 10.3390/cancers16020343. Cancers (Basel). 2024. PMID: 38254832 Free PMC article.
-
Adamts10 controls transforming growth factor β family signaling that contributes to retinal ganglion cell development.Front Mol Biosci. 2022 Sep 6;9:989851. doi: 10.3389/fmolb.2022.989851. eCollection 2022. Front Mol Biosci. 2022. PMID: 36148008 Free PMC article.
-
Direct Regulation of Alternative Splicing by SMAD3 through PCBP1 Is Essential to the Tumor-Promoting Role of TGF-β.Mol Cell. 2016 Nov 3;64(3):549-564. doi: 10.1016/j.molcel.2016.09.013. Epub 2016 Oct 13. Mol Cell. 2016. PMID: 27746021 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

