Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer
- PMID: 22986532
- PMCID: PMC3527659
- DOI: 10.1038/onc.2012.412
Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer
Abstract
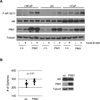
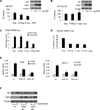
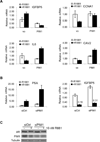
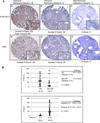
Integration of cellular signaling pathways with androgen receptor (AR) signaling can be achieved through phosphorylation of AR by cellular kinases. However, the kinases responsible for phosphorylating the AR at numerous sites and the functional consequences of AR phosphorylation are only partially understood. Bioinformatic analysis revealed AR serine 213 (S213) as a putative substrate for PIM1, a kinase overexpressed in prostate cancer. Therefore, phosphorylation of AR serine 213 by PIM1 was examined using a phosphorylation site-specific antibody. Wild-type PIM1, but not catalytically inactive PIM1, specifically phosphorylated AR but not an AR serine-to-alanine mutant (S213A). In vitro kinase assays confirmed that PIM1 can phosphorylate AR S213 in a ligand-independent manner and cell type-specific phosphorylation was observed in prostate cancer cell lines. Upon PIM1 overexpression, AR phosphorylation was observed in the absence of hormone and was further increased in the presence of hormone in LNCaP, LNCaP-abl and VCaP cells. Moreover, phosphorylation of AR was reduced in the presence of PIM kinase inhibitors. An examination of AR-mediated transcription showed that reporter gene activity was reduced in the presence of PIM1 and wild-type AR, but not S213A mutant AR. Androgen-mediated transcription of endogenous PSA, Nkx3.1 and IGFBP5 was also decreased in the presence of PIM1, whereas IL6, cyclin A1 and caveolin 2 were increased. Immunohistochemical analysis of prostate cancer tissue microarrays showed significant P-AR S213 expression that was associated with hormone refractory prostate cancers, likely identifying cells with catalytically active PIM1. In addition, prostate cancers expressing a high level of P-AR S213 were twice as likely to be from biochemically recurrent cancers. Thus, AR phosphorylation by PIM1 at S213 impacts gene transcription and is highly prevalent in aggressive prostate cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
PIM1 phosphorylation of the androgen receptor and 14-3-3 ζ regulates gene transcription in prostate cancer.Commun Biol. 2021 Oct 25;4(1):1221. doi: 10.1038/s42003-021-02723-9. Commun Biol. 2021. PMID: 34697370 Free PMC article.
-
Pim1 regulates androgen-dependent survival signaling in prostate cancer cells.Urol Int. 2010;84(2):212-20. doi: 10.1159/000277601. Epub 2010 Mar 4. Urol Int. 2010. PMID: 20215828
-
Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases.J Biol Chem. 2012 Jun 29;287(27):22959-68. doi: 10.1074/jbc.M111.338350. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584579 Free PMC article.
-
PIM1 kinase as a target in prostate cancer: roles in tumorigenesis, castration resistance, and docetaxel resistance.Curr Cancer Drug Targets. 2014;14(2):105-14. doi: 10.2174/1568009613666131126113854. Curr Cancer Drug Targets. 2014. PMID: 24274399 Review.
-
Androgen Receptor and PIM1 Expression in Tumor Tissue of Patients With Triple-negative Breast Cancer.Cancer Genomics Proteomics. 2021 Mar-Apr;18(2):147-156. doi: 10.21873/cgp.20249. Cancer Genomics Proteomics. 2021. PMID: 33608311 Free PMC article. Review.
Cited by
-
PIM-1 kinase: a potential biomarker of triple-negative breast cancer.Onco Targets Ther. 2019 Aug 8;12:6267-6273. doi: 10.2147/OTT.S212752. eCollection 2019. Onco Targets Ther. 2019. PMID: 31496730 Free PMC article.
-
PIM1 phosphorylation of the androgen receptor and 14-3-3 ζ regulates gene transcription in prostate cancer.Commun Biol. 2021 Oct 25;4(1):1221. doi: 10.1038/s42003-021-02723-9. Commun Biol. 2021. PMID: 34697370 Free PMC article.
-
Molecular and cellular mechanisms of castration resistant prostate cancer.Oncol Lett. 2018 May;15(5):6063-6076. doi: 10.3892/ol.2018.8123. Epub 2018 Feb 27. Oncol Lett. 2018. PMID: 29616091 Free PMC article. Review.
-
Chrebp regulates the transcriptional activity of androgen receptor in prostate cancer.Tumour Biol. 2014 Aug;35(8):8143-8. doi: 10.1007/s13277-014-2085-8. Epub 2014 May 21. Tumour Biol. 2014. PMID: 24845031
-
Mini-review: androgen receptor phosphorylation in prostate cancer.Am J Clin Exp Urol. 2013 Dec 25;1(1):25-9. eCollection 2013. Am J Clin Exp Urol. 2013. PMID: 25374897 Free PMC article. Review.
References
-
- Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000 Dec 15;60(24):6841–6845. - PubMed
-
- Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, et al. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005 Dec 9;280(49):40916–40924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

