The ablation of EZH2 uncovers its crucial role in rhabdomyosarcoma formation
- PMID: 22983009
- PMCID: PMC3495825
- DOI: 10.4161/cc.22025
The ablation of EZH2 uncovers its crucial role in rhabdomyosarcoma formation
Abstract
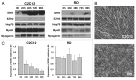
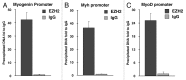
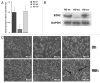
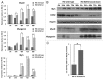
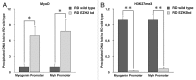
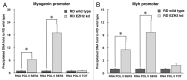
Rhabdomyosarcoma (RMS) is a pediatric tumor that arises from muscle precursor cells. RMS cells express several markers of early myogenic differentiation, but they fail to complete both differentiation program and cell cycle arrest, resulting in uncontrolled proliferation and incomplete myogenesis. Previous studies showed that EZH2, which is involved in both differentiation and cancer progression, is overexpressed in RMS, but a functional binding between its expression and its functional role in tumor formation or progression has not yet been demonstrated. We hypothesized that EZH2 is a key regulator of muscular differentiation program in RMS cells. In this study, we demonstrated that EZH2 directly binds muscle specific genes in RD cells. Silencing of EZH2 promotes the recruitment of a multiprotein complex at muscle-specific promoters, their transcriptional activation and protein expression. Moreover, we demonstrated that EZH2 is directly involved in transcriptional repression of MyoD, the main factor promoting myogenesis. EZH2 ablation induces MyoD activation the recovery of its binding on muscle-specific genes.
Figures
Similar articles
-
Pharmacological inhibition of EZH2 as a promising differentiation therapy in embryonal RMS.BMC Cancer. 2014 Feb 27;14:139. doi: 10.1186/1471-2407-14-139. BMC Cancer. 2014. PMID: 24575771 Free PMC article.
-
Comparison of genome-wide binding of MyoD in normal human myogenic cells and rhabdomyosarcomas identifies regional and local suppression of promyogenic transcription factors.Mol Cell Biol. 2013 Feb;33(4):773-84. doi: 10.1128/MCB.00916-12. Epub 2012 Dec 10. Mol Cell Biol. 2013. PMID: 23230269 Free PMC article.
-
Roles of enhancer of zeste homolog 2: from skeletal muscle differentiation to rhabdomyosarcoma carcinogenesis.Cell Cycle. 2014;13(4):516-27. doi: 10.4161/cc.27921. Epub 2014 Jan 22. Cell Cycle. 2014. PMID: 24496329 Review.
-
The Polycomb group (PcG) protein EZH2 supports the survival of PAX3-FOXO1 alveolar rhabdomyosarcoma by repressing FBXO32 (Atrogin1/MAFbx).Oncogene. 2014 Aug 7;33(32):4173-84. doi: 10.1038/onc.2013.471. Epub 2013 Nov 11. Oncogene. 2014. PMID: 24213577
-
PRC2: an epigenetic multiprotein complex with a key role in the development of rhabdomyosarcoma carcinogenesis.Clin Epigenetics. 2021 Aug 9;13(1):156. doi: 10.1186/s13148-021-01147-w. Clin Epigenetics. 2021. PMID: 34372908 Free PMC article. Review.
Cited by
-
Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area.Nat Neurosci. 2015 Mar;18(3):415-22. doi: 10.1038/nn.3932. Epub 2015 Feb 2. Nat Neurosci. 2015. PMID: 25643298 Free PMC article.
-
Pharmacological inhibition of EZH2 as a promising differentiation therapy in embryonal RMS.BMC Cancer. 2014 Feb 27;14:139. doi: 10.1186/1471-2407-14-139. BMC Cancer. 2014. PMID: 24575771 Free PMC article.
-
Attenuated Epigenetic Suppression of Muscle Stem Cell Necroptosis Is Required for Efficient Regeneration of Dystrophic Muscles.Cell Rep. 2020 May 19;31(7):107652. doi: 10.1016/j.celrep.2020.107652. Cell Rep. 2020. PMID: 32433961 Free PMC article.
-
Pediatric Rhabdomyosarcoma.Crit Rev Oncog. 2015;20(3-4):227-43. doi: 10.1615/critrevoncog.2015013800. Crit Rev Oncog. 2015. PMID: 26349418 Free PMC article. Review.
-
Methyltransferase Inhibition Enables Tgfβ Driven Induction of CDKN2A and B in Cancer Cells.Mol Cell Biol. 2023;43(3):115-129. doi: 10.1080/10985549.2023.2186074. Mol Cell Biol. 2023. PMID: 36941772 Free PMC article.
References
-
- Otten AD, Firpo EJ, Gerber AN, Brody LL, Roberts JM, Tapscott SJ. Inactivation of MyoD-mediated expression of p21 in tumor cell lines. Cell Growth Differ. 1997;8:1151–60. - PubMed
-
- Saab R, Spunt SL, Skapek SX. Chapter 7 - Myogenesis and Rhabdomyosarcoma: The Jekyll and Hyde of Skeletal Muscle. 1sted. Elsevier Inc; 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
