Virus factories: biogenesis and structural design
- PMID: 22978691
- PMCID: PMC7162364
- DOI: 10.1111/cmi.12029
Virus factories: biogenesis and structural design
Abstract
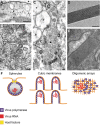
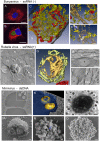
Replication and assembly of many viruses occur in specific intracellular compartments known as 'virus factories'. Our knowledge of the biogenesis and architecture of these unique structures has increased considerably in the last 10 years, due to technical advances in cellular, molecular and structural biology. We now know that viruses build replication organelles, which recruit cell and viral components in a macrostructure in which viruses assemble and mature. Cell membranes and cytoskeleton participate in the biogenesis of these scaffolds and mitochondria are present in many factories, where they might supply energy and other essential factors. New inter-organelle contacts have been visualized within virus factories, whose structure is very dynamic, as it changes over time. There is increasing interest in identifying the factors involved in their biogenesis and functional architecture, and new microscopy techniques are helping us to understand how these complex entities are built and work. In this review, we summarize recent findings on the cell biology, biogenesis and structure of virus factories.
© 2012 Blackwell Publishing Ltd.
Figures
Similar articles
-
Virus assembly factories in a lipid world.Curr Opin Virol. 2016 Jun;18:20-6. doi: 10.1016/j.coviro.2016.02.009. Epub 2016 Mar 15. Curr Opin Virol. 2016. PMID: 26985879 Review.
-
Virus factories: associations of cell organelles for viral replication and morphogenesis.Biol Cell. 2005 Feb;97(2):147-72. doi: 10.1042/BC20040058. Biol Cell. 2005. PMID: 15656780 Free PMC article. Review.
-
Infection cycles of large DNA viruses: emerging themes and underlying questions.Virology. 2014 Oct;466-467:3-14. doi: 10.1016/j.virol.2014.05.037. Epub 2014 Jul 2. Virology. 2014. PMID: 24996494 Review.
-
Flaviviridae Replication Organelles: Oh, What a Tangled Web We Weave.Annu Rev Virol. 2015 Nov;2(1):289-310. doi: 10.1146/annurev-virology-100114-055007. Annu Rev Virol. 2015. PMID: 26958917 Review.
-
A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication.Adv Virus Res. 2007;70:101-82. doi: 10.1016/S0065-3527(07)70004-0. Adv Virus Res. 2007. PMID: 17765705 Free PMC article. Review.
Cited by
-
The cell biology of Tobacco mosaic virus replication and movement.Front Plant Sci. 2013 Feb 11;4:12. doi: 10.3389/fpls.2013.00012. eCollection 2013. Front Plant Sci. 2013. PMID: 23403525 Free PMC article.
-
Saccharomyces cerevisiae as a Model for Studying Human Neurodegenerative Disorders: Viral Capsid Protein Expression.Int J Mol Sci. 2023 Dec 7;24(24):17213. doi: 10.3390/ijms242417213. Int J Mol Sci. 2023. PMID: 38139041 Free PMC article. Review.
-
The evolutionary ecology of molecular replicators.R Soc Open Sci. 2016 Aug 3;3(8):160235. doi: 10.1098/rsos.160235. eCollection 2016 Aug. R Soc Open Sci. 2016. PMID: 27853598 Free PMC article.
-
SARS-CoV-2 and mitochondrial health: implications of lifestyle and ageing.Immun Ageing. 2020 Nov 9;17(1):33. doi: 10.1186/s12979-020-00204-x. Immun Ageing. 2020. PMID: 33292333 Free PMC article. Review.
-
Subcellular organization of viral particles during maturation of nucleus-forming jumbo phage.Sci Adv. 2022 May 6;8(18):eabj9670. doi: 10.1126/sciadv.abj9670. Epub 2022 May 4. Sci Adv. 2022. PMID: 35507660 Free PMC article.
References
-
- Bandea, C.I. (2009) The origin and evolution of viruses as molecular organisms. Nature Precedings [WWW document]. URL http://hdl.handle.net/10101/npre.2009.3886.1
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

