Route of primary HTLV-1 infection regulates HTLV-1 distribution in reservoir organs of infected mice
- PMID: 22977475
- PMCID: PMC3440678
- DOI: 10.3892/etm.2010.179
Route of primary HTLV-1 infection regulates HTLV-1 distribution in reservoir organs of infected mice
Abstract
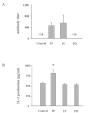
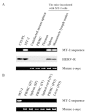
Human T-cell leukemia virus type-1 (HTLV-1) causes adult T-cell leukemia and HTLV-1-associated myelo-pathy/tropical spastic paraparesis. HTLV-1 is mainly transmitted through blood transfusion and breastfeeding, but viral proliferation in the body in vivo shortly after transmission is not well understood. To investigate whether the route of infection influences the early stages of viral proliferation, we inoculated BALB/c mice with MT-2 cells, an HTLV-1-producing human T-cell line, via different routes, and evaluated the proviral load and humoral immune responses. One month after infection, the provirus was detected in most organs of the mice infected intraperitoneally, and substantial proviral loads were detected in the peripheral blood and secondary lymphoid organs. In contrast, the mice infected intravenously and orally showed low proviral loads, and the provirus distribution was limited to the spinal cord among the intravenously inoculated mice and to the liver among the perorally inoculated mice. Mice infected intraperitoneally also exhibited higher interleukin-2 production than the mice infected intravenously or orally, or than the uninfected control mice, while anti-HTLV-1 antibody titers were comparable between the mice infected intraperitoneally and intravenously. These results demonstrate that the route of primary HTLV-1 infection influences the establishment of HTLV-1-infected cell proliferation and the cell reservoir in mice.
Figures
Similar articles
-
De novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice.J Virol. 2006 Nov;80(21):10683-91. doi: 10.1128/JVI.01009-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943297 Free PMC article.
-
Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats.J Virol. 1998 Sep;72(9):7289-93. doi: 10.1128/JVI.72.9.7289-7293.1998. J Virol. 1998. PMID: 9696824 Free PMC article.
-
Transmission of human T-cell leukemia virus type I from a patient with HTLV-I associated myelopathy/tropical spastic paraparesis and an asymptomatic carrier to rabbits.Arch Virol. 1991;118(3-4):235-45. doi: 10.1007/BF01314033. Arch Virol. 1991. PMID: 1712582
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
-
Transcriptional and Epigenetic Regulatory Mechanisms Affecting HTLV-1 Provirus.Viruses. 2016 Jun 16;8(6):171. doi: 10.3390/v8060171. Viruses. 2016. PMID: 27322309 Free PMC article. Review.
Cited by
-
The effect of HTLV1 infection on inflammatory and oxidative parameters in the liver, kidney, and pancreases of BALB/c mice.Physiol Rep. 2022 Apr;10(7):e15243. doi: 10.14814/phy2.15243. Physiol Rep. 2022. PMID: 35373925 Free PMC article.
References
-
- Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. - PubMed
-
- Grant C, Barmak K, Alefantis T, Yao J, Jacobson S, Wigdahl B. Human T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J Cell Physiol. 2002;190:133–159. - PubMed
-
- Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 1984;46:245–253. - PubMed
-
- Okayama A, Stuver S, Matsuoka M, Ishizaki J, Tanaka G, Kubuki Y, Mueller N, Hsieh CC, Tachibana N, Tsubouchi H. Role of HTLV-1 proviral DNA load and clonality in the development of adult T-cell leukemia/lymphoma in asymptomatic carriers. Int J Cancer. 1984;110:621–625. - PubMed
-
- Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S, Osame M. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol. 2001;7:228–234. - PubMed
LinkOut - more resources
Full Text Sources
