Pancreatic stellate cells: a starring role in normal and diseased pancreas
- PMID: 22973234
- PMCID: PMC3428781
- DOI: 10.3389/fphys.2012.00344
Pancreatic stellate cells: a starring role in normal and diseased pancreas
Abstract
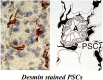
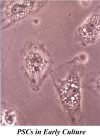
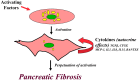
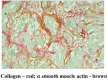
While the morphology and function of cells of the exocrine and endocrine pancreas have been studied over several centuries, one important cell type in the gland, the pancreatic stellate cell (PSC), had remained undiscovered until as recently as 20 years ago. Even after its first description in 1982, it was to be another 16 years before its biology could begin to be studied, because it was only in 1998 that methods were developed to isolate and culture PSCs from rodent and human pancreas. PSCs are now known to play a critical role in pancreatic fibrosis, a consistent histological feature of two major diseases of the pancreas-chronic pancreatitis and pancreatic cancer. In health, PSCs maintain normal tissue architecture via regulation of the synthesis and degradation of extracellular matrix (ECM) proteins. Recent studies have also implied other functions for PSCs as progenitor cells, immune cells or intermediaries in exocrine pancreatic secretion in humans. During pancreatic injury, PSCs transform from their quiescent phase into an activated, myofibroblast-like phenotype that secretes excessive amounts of ECM proteins leading to the fibrosis of chronic pancreatitis and pancreatic cancer. An ever increasing number of factors that stimulate and/or inhibit PSC activation via paracrine and autocrine pathways are being identified and characterized. It is also now established that PSCs interact closely with pancreatic cancer cells to facilitate cancer progression. Based on these findings, several therapeutic strategies have been examined in experimental models of chronic pancreatitis as well as pancreatic cancer, in a bid to inhibit/retard PSC activation and thereby alleviate chronic pancreatitis or reduce tumor growth in pancreatic cancer. The challenge that remains is to translate these pre-clinical developments into clinically applicable treatments for patients with chronic pancreatitis and pancreatic cancer.
Keywords: chronic pancreatic; desmoplastic reaction; pancreatic cancer; pancreatic fibrosis; review; stellate cells.
Figures

Similar articles
-
The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells.Antioxid Redox Signal. 2011 Nov 15;15(10):2711-22. doi: 10.1089/ars.2011.4079. Epub 2011 Aug 12. Antioxid Redox Signal. 2011. PMID: 21728885 Review.
-
Pancreatic stellate cell: Pandora's box for pancreatic disease biology.World J Gastroenterol. 2017 Jan 21;23(3):382-405. doi: 10.3748/wjg.v23.i3.382. World J Gastroenterol. 2017. PMID: 28210075 Free PMC article. Review.
-
Pancreatic stellate cells--multi-functional cells in the pancreas.Pancreatology. 2013 Mar-Apr;13(2):102-5. doi: 10.1016/j.pan.2012.12.058. Pancreatology. 2013. PMID: 23561965 Review.
-
Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis.Clin Gastroenterol Hepatol. 2009 Nov;7(11 Suppl):S48-54. doi: 10.1016/j.cgh.2009.07.038. Clin Gastroenterol Hepatol. 2009. PMID: 19896099 Review.
-
Pancreatic stellate cell: physiologic role, role in fibrosis and cancer.Curr Opin Gastroenterol. 2015 Sep;31(5):416-23. doi: 10.1097/MOG.0000000000000196. Curr Opin Gastroenterol. 2015. PMID: 26125317 Review.
Cited by
-
Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma.Cell Death Dis. 2022 Oct 25;13(10):897. doi: 10.1038/s41419-022-05351-1. Cell Death Dis. 2022. PMID: 36284087 Free PMC article. Review.
-
Human pancreatic islet-derived stromal cells reveal combined features of mesenchymal stromal cells and pancreatic stellate cells.Stem Cell Res Ther. 2024 Oct 8;15(1):351. doi: 10.1186/s13287-024-03963-2. Stem Cell Res Ther. 2024. PMID: 39380125 Free PMC article.
-
Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products.Int J Mol Sci. 2023 Nov 2;24(21):15906. doi: 10.3390/ijms242115906. Int J Mol Sci. 2023. PMID: 37958889 Free PMC article. Review.
-
Global targetome analysis reveals critical role of miR-29a in pancreatic stellate cell mediated regulation of PDAC tumor microenvironment.BMC Cancer. 2020 Jul 13;20(1):651. doi: 10.1186/s12885-020-07135-2. BMC Cancer. 2020. PMID: 32660466 Free PMC article.
-
Ex Vivo Tumor-on-a-Chip Platforms to Study Intercellular Interactions within the Tumor Microenvironment.Adv Healthc Mater. 2019 Feb;8(4):e1801198. doi: 10.1002/adhm.201801198. Epub 2018 Dec 5. Adv Healthc Mater. 2019. PMID: 30516355 Free PMC article. Review.
References
-
- Ammori B. J., Leeder P. C., King R. F., Barclay G. R., Martin I. G., Larvin M., McMahon M. J. (1999). Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J. Gastrointest. Surg. 3, 252–262 10.1016/S1091-255X(99)80067-5 - DOI - PubMed
-
- Andoh A., Takaya H., Saotome T., Shimada M., Hata K., Araki Y., Nakamura F., Shintani Y., Fujiyama Y., Bamba T. (2000). Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology 119, 211–219 - PubMed
-
- Aoki H., Ohnishi H., Hama K., Ishijima T., Satoh Y., Hanatsuka K., Ohashi A., Wada S., Miyata T., Kita H., Yamamoto H., Osawa H., Sato K., Tamada K., Yasuda H., Mashima H., Sugano K. (2006). Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am. J. Physiol. Cell Physiol. 290, C1100–C1108 10.1152/ajpcell.00465.2005 - DOI - PubMed
-
- Aoki H., Ohnishi H., Hama K., Shinozaki S., Kita H., Osawa H., Yamamoto H., Sato K., Tamada K., Sugano K. (2007). Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to proinflammatory cytokines. Am. J. Physiol. Cell Physiol. 292, C259–C268 10.1152/ajpcell.00030.2006 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

