Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow-responder adult patients
- PMID: 22972122
- PMCID: PMC11538912
- DOI: 10.1002/14651858.CD008516.pub2
Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow-responder adult patients
Abstract
Background: The standard length of peginterferon plus ribavirin treatment for chronic hepatitis C virus (HCV) genotype 1 infected patients is 48 weeks. However, the number of patients demonstrating a sustained virological response is not high. In order to improve sustained virological response, extending the length of the treatment period has been suggested.
Objectives: To study the benefits and harms of extended 72-week treatment in comparison with 48-week treatment with peginterferon plus ribavirin in patients with chronic HCV genotype 1 infection who have shown a slow antiviral response.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and LILACS until November 2011. We identified further trials by reviewing reference lists and contacting principal authors.
Selection criteria: Trials were eligible for this review if they included patients infected with hepatitis C virus genotype 1 who had a slow antiviral response, and if those patients were randomised to completing 72 weeks versus 48 weeks of treatment with pegylated interferon and ribavirin.
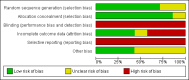
Data collection and analysis: Two authors independently assessed the trials for risk of bias, and extracted the data. The primary outcomes were overall mortality, liver-related mortality, and liver-related morbidity. We extracted data separately according to two definitions of slow responders: 1) patients with ≥ 2 log viral reduction but still detectable HCV RNA after 12 weeks of treatment and undetectable HCV RNA after 24 weeks of treatment; 2) patients with detectable HCV RNA after four weeks of treatment. We calculated risk ratios from individual trials as well as in the meta-analyses of trials.
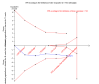
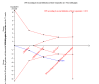
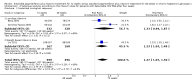
Main results: We included seven trials with 1369 participants. All trials had high risk of bias. Five trials used our first definition of slow responders, and three other trials (including one that used both definitions) used the second definition. None of the included trials mentioned our primary outcomes. However, regarding the secondary outcomes, extension of the treatment period to 72 weeks increased the sustained virological response according to both definitions (71/217 (32.7%) versus 52/194 (26.8%); risk ratio (RR) 1.43, 95% confidence interval (CI) 1.07 to 1.92, P = 0.02, I(2) = 8%; and 265/499 (53.1%) versus 207/496 (41.7%); RR 1.27, 95% CI 1.07 to 1.50, P = 0.006, I(2) = 38%), with a risk difference of 0.11 and calculated number needed to treat of nine. The end of treatment response was not significantly different between the two treatment groups. The number of participants who relapsed virologically was found to be lower in the groups that had been treated for 72 weeks using both definitions (27/84 (32.1%) versus 46/91 (50.5%); RR 0.59, 95% CI 0.40 to 0.86, P = 0.007, I(2) = 18%, 3 trials; and 85/350 (24.3%) versus 146/353 (41.4%); RR 0.59, 95% CI 0.47, 0.73, P < 0.000001, I(2) = 0%, 3 trials). The length of treatment did not significantly affect the adherence (247/279 (88.5%) versus 252/274 (92.0%); RR 0.95, 95% CI 0.84 to 1.07, P = 0.42, I(2) = 69%, 3 trials). In the single trial that reported adverse events, no significant difference was seen between the two treatment groups.
Authors' conclusions: This review demonstrates higher a proportion of sustained virological response after extension of treatment from 48 weeks to 72 weeks in HCV genotype 1 infected patients in whom HCV RNA was still detectable but decreased by ≥ 2 log after 12 weeks and became negative after 24 weeks of treatment, and in patients with detectable HCV RNA after four weeks of treatment with peginterferon plus ribavirin. The observed intervention effects can be caused by both systematic error (bias) and random errors (play of chance). There was no reporting on mortality and the reporting of clinical outcomes and adverse events was insufficient. More data are needed in order to recommend or reject the policy of extending the treatment period for slow responders.
Conflict of interest statement
None known
Figures









Update of
- doi: 10.1002/14651858.CD008516
Similar articles
-
Peginterferon plus ribavirin versus interferon plus ribavirin for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Feb 28;2014(2):CD005441. doi: 10.1002/14651858.CD005441.pub3. Cochrane Database Syst Rev. 2014. PMID: 24585509 Free PMC article. Review.
-
Peginterferon alpha-2a versus peginterferon alpha-2b for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Feb 28;2014(2):CD005642. doi: 10.1002/14651858.CD005642.pub3. Cochrane Database Syst Rev. 2014. PMID: 24585451 Free PMC article. Review.
-
Aminoadamantanes for chronic hepatitis C.Cochrane Database Syst Rev. 2014 May 3;2014(5):CD010125. doi: 10.1002/14651858.CD010125.pub2. Cochrane Database Syst Rev. 2014. PMID: 24793264 Free PMC article. Review.
-
Nitazoxanide for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Apr 6;2014(4):CD009182. doi: 10.1002/14651858.CD009182.pub2. Cochrane Database Syst Rev. 2014. PMID: 24706397 Free PMC article. Review.
-
Aminoadamantanes versus other antiviral drugs for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD011132. doi: 10.1002/14651858.CD011132.pub2. Cochrane Database Syst Rev. 2014. PMID: 24937404 Free PMC article. Review.
Cited by
-
Interventions for the management of fatigue in adults with a primary brain tumour.Cochrane Database Syst Rev. 2016 Apr 13;4(4):CD011376. doi: 10.1002/14651858.CD011376.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2022 Sep 12;9:CD011376. doi: 10.1002/14651858.CD011376.pub3. PMID: 27074263 Free PMC article. Updated. Review.
-
Decade of optimizing therapy with direct-acting antiviral drugs and the changing profile of patients with chronic hepatitis C.World J Gastroenterol. 2023 Feb 14;29(6):949-966. doi: 10.3748/wjg.v29.i6.949. World J Gastroenterol. 2023. PMID: 36844142 Free PMC article. Review.
-
Interventions for the management of fatigue in adults with a primary brain tumour.Cochrane Database Syst Rev. 2022 Sep 12;9(9):CD011376. doi: 10.1002/14651858.CD011376.pub3. Cochrane Database Syst Rev. 2022. PMID: 36094728 Free PMC article. Review.
-
Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings.World J Gastroenterol. 2015 Feb 14;21(6):1972-81. doi: 10.3748/wjg.v21.i6.1972. World J Gastroenterol. 2015. PMID: 25684966 Free PMC article. Review.
References
References to studies included in this review
Berg 2006 {published data only}
-
- Berg T, Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon‐alfa‐2a plus ribavirin. Gastroenterology 2006;130:1086–97. - PubMed
Buti 2010 {published data only}
-
- Buti M, Lurie Y, Zakharova N, Blokhina N, Horban A, Teuber G, et al. Randomized trial of peginterferon alfa‐2b and ribavirin for 48 or 72 weeks in patients with HCV genotype 1 and slow virologic response. Hepatology 2010;52(4):1201‐7. - PubMed
Lee 2012 {published data only}
-
- Lee SS, Sherman M, Ramji A, Greenbloom S, Elkashab M, Pluta H, et al. Randomised clinical trial: the efficacy of treatment, guided by a shorter duration of response, using peginterferon alfa‐2a plus ribavirin for hepatitis C virus other than genotypes 2 or 3. Alimentary Pharmacolology and Therapuetics 2012;35(1):37‐47. - PubMed
Liu 2011 {published and unpublished data}
-
- Liu C‐H, Liang C‐C, Liu C‐J, Lin C‐L, Yang S‐S, Hsu S‐J, et al. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks for patients with chronic hepatitis C virus genotype 1 infection having shown slow antiviral response. Hepatology International. 2011; Vol. 21st Conference of the Asian Pacific Association for the Study of the Liver, APASL 2011.
Mangia 2008 {published data only}
-
- Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci G, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology 2008;47:43‐50. - PubMed
Pearlman 2007 {published data only}
-
- Pearlman B, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1–infected slow responders. Hepatology 2007;46:1688‐94. - PubMed
Sanchez‐Tapias 2006 {published data only}
-
- Sánchez‐Tapias J, Diado M, Escartín P, Enríquez J, Romero‐Gómez M, Bárcena R, et al. Peginterferon‐alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 2006;131:451–60. - PubMed
References to studies excluded from this review
Ferenci 2010 {published data only}
-
- Ferenci P, Laferl H, Scherzer TM, Maieron A, Hofer H, Stauber R, et al. Peginterferon alfa‐2a/ribavirin for 48 or 72 weeks in hepatitis C types 1and 4 patients with slow virologic response. Gastroenterology 2010;138:503‐12. - PubMed
Ide 2009 {published data only}
-
- Ide T, Hino T, Ogata K, Miyajima I, Kuwahara R, Kuhara K, et al. A randomized study of extended treatment with peginterferon α‐2b plus ribavirin based on time to HCV RNA negative – status in patients with genotype 1b chronic hepatitis C. American Journal of Gastroenterology 2009;104:70‐5. - PubMed
Miyase 2010 {published data only}
-
- Miyase S, Ogata K, Haraoka K, Morishita Y, Fujiyama S. The efficacy of prolonging treatment with peginterferon alfa‐2b and ribavirin to 72 weeks in chronic hepatitis C genotype 1 patients HCV RNA positive at week 8 but negative at week 12. Acta Hepatologica Japonica 2010;51:48‐50.
Nagaki 2009 {published data only}
-
- Nagaki M, Shimizu M, Sugihara J, Tomita E, Sano C, Naiki T, et al. Clinical trial: extended treatment duration of peginterferon alpha2b plus ribavirin for 72 and 96 weeks in hepatitis C genotype 1‐infected late responders. Alimentary Pharmacology and Therapeutics 2009;30:343–51. - PubMed
References to ongoing studies
Sarrazin 2010 {published data only}
-
- Sarrazin C, Schwendy S, Möller B, Dikopoulos N, Buggisch P, Encke J, et al. Completely individualized treatment durations (24, 30, 36, 42, 48, 60 or 72 weeks) with peginterferon‐alfa‐2b and ribavirin in HCV genotype 1‐infected patients (INDIV‐2 study). Journal of Hepatology 2010;52:S25‐S26.
Additional references
Abergel 2004
-
- Abergel A, Darcha C, Chevallier M, Ughetto S, Henquell C, Pol S, et al. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus‐related severe fibrosis. European Journal of Gastroenterology and Hepatology 2004;16(11):1219‐27. - PubMed
Alavian 2011
Awad 2010
-
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha‐2a is associated with higher sustained virological response than peginterferon alfa‐2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51:1176‐84. - PubMed
Berg 2009
-
- Berg T, Weich V, Teuber G, Klinker H, Moller B, Rasenack J, et al. Individualized treatment strategy according to early viral kinetics in hepatitis C virus type 1‐infected patients. Hepatology 2009;50:369‐77. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Brok 2010
Buti 2003
-
- Buti M, Valdes A, Sanchez‐Avila F, Esteban R, Lurie Y. Extending combination therapy with peginterferon alfa‐2b plus ribavirin for genotype 1chronic hepatitis C late responders: a report of 9 cases. Hepatology 2003;37:1226‐7. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ (accessed January 2011).
Davis 2003
-
- Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003;38:645‐52. - PubMed
DeMets 1987
-
- DeMets DL. Practical aspects in data monitoring: a brief review. Statistics in Medicine 1987;6(7):753‐60. - PubMed
Di Martino 2011
-
- Martino V, Richou C, Cervoni JP, Sanchez‐Tapias JM, Jensen DM, Mangia A, et al. Response‐guided peg‐interferon plus ribavirin treatment duration in chronic hepatitis C: Meta‐analyses of randomized, controlled trials and implications for the future. Hepatology 2011;54(3):789‐800. - PubMed
DiBisceglie 2011
Drusano 2004
-
- Drusano GL, Preston SL. A 48‐week duration of therapy with pegylated interferon alpha 2b plus ribavirin may be too short to maximize long‐term response among patients infected with genotype‐1 hepatitis C virus. Journal of Infectious Diseases 2004;189:964‐70. - PubMed
Egger 1997
El‐Serag 2003
-
- El‐Serag H, Davila J, Petersen N, McGlynn K. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Annals of Internal Medicine 2003;139:817‐23. - PubMed
Farnik 2010
-
- Farnik H, Lange CM, Sarrazin C, Kronenberger B, Zeuzem S, Herrmann E. Meta‐analysis shows extended therapy improves response of patients with chronic hepatitis C virus genotype 1 infection. Clinical Gastroenterology and Hepatology 2010;8(10):884‐90. - PubMed
Ferenci 2005
-
- Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa‐2a (40 KD)/ribavirin. Journal of Hepatology 2005;43:425‐33. - PubMed
Fried 2002
-
- Fried MW, Shiffman M, Reddy KR, Smith C, Marinos G, Goncales FL, et al. Pefintereron alfa‐2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine 2002;347:975‐82. - PubMed
Gamble 2005
-
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. - PubMed
Gevers 2011
-
- Gevers TJ, Slavenburg S, Oijen MG, Drenth JP. Treatment extension benefits HCV genotype 1 patients without rapid virological response: a systematic review. Netherlands Journal of Medicine 2011;69(5):216‐21. - PubMed
Ghany 2009
Gluud 2012
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 5. Art. No.: LIVER.
Hadziyannis 2004
-
- Hadziyannis S, Sette H Jr, Morgan T, Balan V, Diago M, Marcellin P, et al. Peginterferon‐2a and ribavirin combination therapy in chronic hepatitis C. Annals of Internal Medicine 2004;140:346‐55. - PubMed
Hezode 2009
-
- Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. New England Journal of Medicine 2009;360:1839‐50. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Innes 2011
-
- Innes HA, Hutchinson SJ, Allen S, Bhattacharyya D, Bramley P, Delahooke TE, et al. Excess liver‐related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology 2011;54(5):1547‐58. - PubMed
Jensen 2006
-
- Jensen M, Morgan T, Marcellin P, Pockros P, Reddy K, Hadziyannis S, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon‐2a (40 kd)/ribavirin therapy. Hepatology 2006;43:954‐60. - PubMed
Khuroo 2004
-
- Khuroo M, Khuroo M, Dahab S. Meta‐analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Alimentary Pharmacology and Therapeutics 2004;20:931‐8. - PubMed
Kim 2009
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lauer 2001
-
- Lauer G, Walker A. Hepatits C virus infection. New England Journal of Medicine 2001;345:41‐52. - PubMed
Manns 2001
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958‐65. - PubMed
McHutchison 2009
-
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa‐2b or alfa‐2a with ribavirin for treatment of hepatitis C infection. New England Journal of Medicine 2009;361:580‐93. - PubMed
McHutchison 2009b
-
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. New England Journal of Medicine 2009;360:1827‐38. - PubMed
Michaels 2010
-
- Michaels AJ, Nelson DR. New therapies in the management of hepatitis C virus. Current Opinions in Gastroenterology 2010;26:196‐201. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Morgan 2010
Parikh 2010
-
- Parikh M, Singh A, Sood G. Extended treatment duration for treatment naive chronic hepatitis C genotype 1 late viral responders: a meta‐analysis comparing 48 weeks vs 72 weeks of pegylated interferon and ribavirin. Journal of Viral Hepatitis 2010; Vol. 18, issue 4:e99‐e103. [DOI: 10.1111/j.1365-2893.2010.01374.x] - DOI - PubMed
Poynard 1995
-
- Poynard T, Bedossa P, Chevallier M, Mathurin P, Lemonnier C, Trepo C, et al. A comparison of three interferon alfa‐2b regimens for the long‐term treatment of chronic non‐A, non‐B hepatitis. Multicenter study group. New England Journal of Medicine 1995;332:1457‐62. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Scherzer 2009
-
- Scherzer TM, Kerschner H, Beinhardt S, Rutter K, Hofer H, Steindl‐Munda P, et al. Week 8 HCV‐RNA is the optimal predictor of relapse in non‐RVR patients with genotype 1/4 randomised to 48 or 72 weeks PEG‐IFN alfa‐2A plus RBV. Journal of Hepatology 2009;50(Suppl 1):S225.
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Theodore 2006
Thomas 2005
-
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clinical Liver Diseases 2005;9:383‐98. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 13 June 2012).
Ueno 2009
-
- Ueno Y, Sollano J, Farrell G. Prevention of hepatocellular carcinoma complicating chronic hepatitis C. Journal of Gastroenterology and Hepatology 2009;24:531‐6. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

