Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease
- PMID: 22972099
- PMCID: PMC4170910
- DOI: 10.1002/14651858.CD006829.pub2
Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease
Abstract
Background: Both inhaled steroids (ICS) and long-acting beta(2)-agonists (LABA) are used in the management of chronic obstructive pulmonary disease (COPD). This updated review compared compound LABA plus ICS therapy (LABA/ICS) with the LABA component drug given alone.
Objectives: To assess the efficacy of ICS and LABA in a single inhaler with mono-component LABA alone in adults with COPD.
Search methods: We searched the Cochrane Airways Group Specialised Register of trials. The date of the most recent search was November 2011.
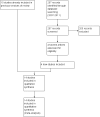
Selection criteria: We included randomised, double-blind controlled trials. We included trials comparing compound ICS and LABA preparations with their component LABA preparations in people with COPD.
Data collection and analysis: Two authors independently assessed study risk of bias and extracted data. The primary outcomes were exacerbations, mortality and pneumonia, while secondary outcomes were health-related quality of life (measured by validated scales), lung function, withdrawals due to lack of efficacy, withdrawals due to adverse events and side-effects. Dichotomous data were analysed as random-effects model odds ratios or rate ratios with 95% confidence intervals (CIs), and continuous data as mean differences and 95% CIs. We rated the quality of evidence for exacerbations, mortality and pneumonia according to recommendations made by the GRADE working group.
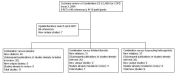
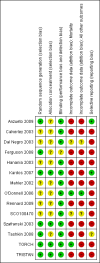
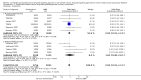
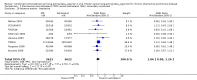
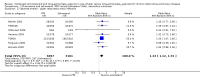
Main results: Fourteen studies met the inclusion criteria, randomising 11,794 people with severe COPD. We looked at any LABA plus ICS inhaler (LABA/ICS) versus the same LABA component alone, and then we looked at the 10 studies which assessed fluticasone plus salmeterol (FPS) and the four studies assessing budesonide plus formoterol (BDF) separately. The studies were well-designed with low risk of bias for randomisation and blinding but they had high rates of attrition, which reduced our confidence in the results for outcomes other than mortality.Primary outcomes There was low quality evidence that exacerbation rates in people using LABA/ICS inhalers were lower in comparison to those with LABA alone, from nine studies which randomised 9921 participants (rate ratio 0.76; 95% CI 0.68 to 0.84). This corresponds to one exacerbation per person per year on LABA and 0.76 exacerbations per person per year on ICS/LABA. Our confidence in this effect was limited by statistical heterogeneity between the results of the studies (I(2) = 68%) and a risk of bias from the high withdrawal rates across the studies. When analysed as the number of people experiencing one or more exacerbations over the course of the study, FPS lowered the odds of an exacerbation with an odds ratio (OR) of 0.83 (95% CI 0.70 to 0.98, 6 studies, 3357 participants). With a risk of an exacerbation of 47% in the LABA group over one year, 42% of people treated with LABA/ICS would be expected to experience an exacerbation. Concerns over the effect of reporting biases led us to downgrade the quality of evidence for this effect from high to moderate.There was no significant difference in the rate of hospitalisations (rate ratio 0.79; 95% CI 0.55 to 1.13, very low quality evidence due to risk of bias, statistical imprecision and inconsistency). There was no significant difference in mortality between people on combined inhalers and those on LABA, from 10 studies on 10,680 participants (OR 0.92; 95% CI 0.76 to 1.11, downgraded to moderate quality evidence due to statistical imprecision). Pneumonia occurred more commonly in people randomised to combined inhalers, from 12 studies with 11,076 participants (OR 1.55; 95% CI 1.20 to 2.01, moderate quality evidence due to risk of bias in relation to attrition) with an annual risk of around 3% on LABA alone compared to 4% on combination treatment. There were no significant differences between the results for either exacerbations or pneumonia from trials adding different doses or types of inhaled corticosteroid.Secondary outcomes ICS/LABA was more effective than LABA alone in improving health-related quality of life measured by the St George's Respiratory Questionnaire (1.58 units lower with FPS; 2.69 units lower with BDF), dyspnoea (0.09 units lower with FPS), symptoms (0.07 units lower with BDF), rescue medication (0.38 puffs per day fewer with FPS, 0.33 puffs per day fewer with BDF), and forced expiratory volume in one second (FEV(1)) (70 mL higher with FPS, 50 mL higher with BDF). Candidiasis (OR 3.75) and upper respiratory infection (OR 1.32) occurred more frequently with FPS than SAL. We did not combine adverse event data relating to candidiasis for BDF studies as the results were very inconsistent.
Authors' conclusions: Concerns over the analysis and availability of data from the studies bring into question the superiority of ICS/LABA over LABA alone in preventing exacerbations. The effects on hospitalisations were inconsistent and require further exploration. There was moderate quality evidence of an increased risk of pneumonia with ICS/LABA. There was moderate quality evidence that treatments had similar effects on mortality. Quality of life, symptoms score, rescue medication use and FEV(1) improved more on ICS/LABA than on LABA, but the average differences were probably not clinically significant for these outcomes. To an individual patient the increased risk of pneumonia needs to be balanced against the possible reduction in exacerbations.More information would be useful on the relative benefits and adverse event rates with combination inhalers using different doses of inhaled corticosteroids. Evidence from head-to-head comparisons is needed to assess the comparative risks and benefits of the different combination inhalers.
Conflict of interest statement
The authors who have been involved in this review have done so without any known conflicts of interest. None of the authors are considered a paid consultant by any pharmaceutical company which produces agents discussed in this review.
Figures


















































Update of
-
Combined corticosteroid and long-acting beta-agonist in one inhaler versus long-acting beta-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006829. doi: 10.1002/14651858.CD006829. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Sep 12;(9):CD006829. doi: 10.1002/14651858.CD006829.pub2. PMID: 17943918 Updated. Review.
Comment in
-
ACP Journal Club. Review: corticosteroid plus LABA inhalers, vs LABAs alone, reduce morbidity in COPD.Ann Intern Med. 2013 Feb 19;158(4):JC9. doi: 10.7326/0003-4819-158-4-201302190-02009. Ann Intern Med. 2013. PMID: 23420259 No abstract available.
Similar articles
-
Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2023 Dec 6;12(12):CD011600. doi: 10.1002/14651858.CD011600.pub3. Cochrane Database Syst Rev. 2023. PMID: 38054551 Free PMC article. Review.
-
Combined corticosteroid and long-acting beta₂-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Nov 10;2013(11):CD003794. doi: 10.1002/14651858.CD003794.pub4. Cochrane Database Syst Rev. 2013. PMID: 24214176 Free PMC article. Review.
-
Combination inhaled steroid and long-acting beta2-agonist versus tiotropium for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 May 31;2013(5):CD007891. doi: 10.1002/14651858.CD007891.pub3. Cochrane Database Syst Rev. 2013. PMID: 23728670 Free PMC article. Review.
-
Long-acting beta2-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Oct 15;2013(10):CD010177. doi: 10.1002/14651858.CD010177.pub2. Cochrane Database Syst Rev. 2013. PMID: 24127118 Free PMC article. Review.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
Cited by
-
The impact of adherence to inhaled drugs on 5-year survival in COPD patients: a time dependent approach.Pharmacoepidemiol Drug Saf. 2016 Nov;25(11):1295-1304. doi: 10.1002/pds.4059. Epub 2016 Jul 11. Pharmacoepidemiol Drug Saf. 2016. PMID: 27396695 Free PMC article.
-
COPD exacerbations by disease severity in England.Int J Chron Obstruct Pulmon Dis. 2016 Apr 1;11:697-709. doi: 10.2147/COPD.S100250. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27099486 Free PMC article.
-
Inhaled corticosteroids in chronic obstructive pulmonary disease: a pro-con perspective.Br J Clin Pharmacol. 2014 Aug;78(2):282-300. doi: 10.1111/bcp.12334. Br J Clin Pharmacol. 2014. PMID: 25099256 Free PMC article. Review.
-
A review of national guidelines for management of COPD in Europe.Eur Respir J. 2016 Feb;47(2):625-37. doi: 10.1183/13993003.01170-2015. Epub 2016 Jan 21. Eur Respir J. 2016. PMID: 26797035 Free PMC article. Review.
-
Changes in definition lead to changes in the clinical characteristics across COPD categories according to GOLD 2017: a national cross-sectional survey in China.Int J Chron Obstruct Pulmon Dis. 2017 Oct 20;12:3095-3102. doi: 10.2147/COPD.S142801. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29118578 Free PMC article.
References
References to studies included in this review
Anzueto 2009 {published data only}
-
- Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. Journal of Chronic Obstructive Pulmonary Disease 2009;6(5):320‐9. - PubMed
Calverley 2003 {published and unpublished data}
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22 [Poster 505].
-
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. - PubMed
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P436. - PubMed
-
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P1587.
-
- Calverley PMA, Olsson H, Symbicort International COPD Study Group. Budesonide/formoterol ina single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 [Poster 418].
Dal Negro 2003 {published data only}
-
- Dal Negro R, Micheletto C, Trevsian F, Tognella S. [A98] Salmeterol and fluticasone 50ug/250ug BiD versus salmeterol 50ug bid and versus placebo in the long term treatment of COPD. Proceedings of the 98th International American Thoracic Society Conference. 2002:http://www.abstracts‐on‐line.com/abstracts/ATS.
-
- Dal Negro RW, Pomari C, Tognella S, Micheletto C. Salmeterol & fluticasone 50 microg/250 microg bid in combination provides a better long‐term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulmonary Pharmacology and Therapeutics 2003;16(4):241‐6. - PubMed
Ferguson 2008 {published data only}
-
- Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respiratory Medicine 2008;102:1099‐108. - PubMed
-
- SCO40043. A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS® 250/50mcg bid with salmeterol DISKUS® 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). http://ctr.gsk.co.uk (accessed 8th April 2008).
Hanania 2003 {published and unpublished data}
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory and Critical Care Medicine 2001;163 Suppl(5):A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P434.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; Arlington, Virginia. 2003:Abstract 1081.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P429.
Kardos 2007 {published and unpublished data}
-
- Kardos P, Wencker M. Combination therapy with salmeterol and fluticasone propionate (SFC) is more effective than salmeterol (SAL) alone in reducing exacerbations of COPD. European Respiratory Journal 2005;26 Suppl 49:Abstract 1944.
-
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2007;175(2):144‐9. - PubMed
-
- SCO30006. A randomised, double‐blind, parallel‐group study to investigate the protective effect of the combination of fluticasone and salmeterol (500/50µg bid via the DISKUS) compared with salmeterol (50µg bid via the DISKUS) on the incidence of moderate to severe exacerbations in patients with severe chronic obstructive pulmonary disease (COPD) (GOLD III/IV). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
-
- Vogelmeier C. Combination therapy with salmeterol and fluticasone propionate (SFC) improves quality of life (QoL) more than salmeterol (SAL) alone in COPD. 42nd Nordic Lung Conference Trondheim. 2005; Vol. 13 Suppl 22.
-
- Vogelmeier CF, Wencker M, Glaab TH, Kardos P. Number needed to treat (NNT) to reduce exacerbations in severe COPD comparing salmeterol/fluticasone propionate (SFC) with salmeterol (SAL) treatment. Proceedings of the American Thoracic Society. 2006:A110.
Mahler 2002 {published and unpublished data}
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD). http://www.abstracts2view.com 2003.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. - PubMed
-
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of Salmeterol (SAL) 50mcg BID and Fluticasone Propionate (FP) 500mcg BID individually and in combination as Salmeterol 50mcg/Fluticasone Propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
-
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22 Suppl 45:51s.
-
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnea and fatigue [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93 [Poster 308].
O'Donnell 2006 {published and unpublished data}
-
- O'Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006;130(3):647‐56. - PubMed
-
- SCO40030. A randomized, double‐blind, placebo‐controlled, parallel group clinical trial evaluating the effect of the fluticasone propionate/salmeterol combination product 250/50mcg bid via DISKUS and salmeterol 50mcg bid via DISKUS on lung hyperinflation in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Rennard 2009 {published data only}
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease. Results from a 1‐year randomized controlled clinical trial. Drugs 2009;69(5):549‐65. - PMC - PubMed
SCO100470 {unpublished data only}
-
- SCO100470. A multicentre, randomised, double‐blind, parallel group, 24‐week study to compare the effect of the salmeterol/fluticasone propionate combination product 50/250mcg, with salmeterol 50mcg both delivered twice daily via the DISKUS/ACCUHALER inhaler on lung function and dyspnoea in subjects with Chronic Obstructive Pulmonary Disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2006.
Szafranski 2003 {published and unpublished data}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD [Abstract]. Thorax 2002;57 Suppl III:iii43.
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22 [Poster 505].
-
- Calverley P, Pauwels R, Lofdahl CG, Svensson K, Higenbottam T, L‐G Carlsson, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. - PubMed
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax. BTS Winter meeting 2002:S145.
-
- Calverley PMA, Szafranski W, Andersson A. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD. European Respiratory Journal 2005;26 Suppl 49:Poster 1917.
Tashkin 2008 {published data only}
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease. Results of a 6‐month randomized clinical trial. Drugs 2008;68(14):1975‐2000. - PubMed
TORCH {published and unpublished data}
-
- Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. - PubMed
-
- Calverley PMA, Celli B, Ferguson G, Jenkins C, Jones PW, Pride NB, et al. Baseline characteristics of the first 5,000 COPD patients enrolled in the TORCH survival study. European Respiratory Journal 2003;22 Suppl 45:578s.
-
- Celli B, Calverley PMA, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves health status, reduces exacerbations and improves lung function over three years. European Respiratory Journal 2006;28 Suppl 50:34s.
-
- Ferguson GT, Calverley PMA, Anderson JA, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years. European Respiratory Journal 2006;28 Suppl 50:34s.
-
- Jenkins CR, Calverley PMA, Celli B, Ferguson G, Jones PW, Pride N, et al. Seasonal Patterns of Exacerbation Rates in the TORCH Survival Study. http://www.abstracts2view.com 2007:A839.
TRISTAN {published and unpublished data}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD. http://www.abstracts2view.com 2003:A035 [Poster D50].
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components. Proceedings of the 98th International American Thoracic Society Conference http://www.abstracts‐on‐line.com/abstracts/ATS. 2002:A98 [Poster 306].
-
- Calverly PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20 Suppl 38:242 [P1572].
-
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 [Poster 503].
References to studies excluded from this review
Aaron 2007 {published data only}
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2007;146(8):545‐55. - PubMed
Bourbeau 2007 {published data only}
Cukier 2007 {published data only}
-
- Cukier A, Ferreira CAS, Stelmach R, Ribeiro M, Cortopassi F, Calverley PMA. The effect of bronchodilators and oxygen alone and in combination on self‐paced exercise performance in stable COPD. Respiratory Medicine 2007;101(4):743‐53. - PubMed
Golabi 2006 {published data only}
-
- Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract].. European Respiratory Journal 2006;28 Suppl 50:33s.
Haque 2006 {published data only}
-
- Haque RA, Torrego A, Essilfie‐Quaye S, Kharitonov SA, Johnson M, Adcock IM, et al. Effect of salmeterol and fluticasone on glucocorticoid receptor translocation in sputum macrophages and peripheral blood mononuclear cells from patients with chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2006:A848.
INSPIRE {published data only}
-
- GlaxoSmithKline (SCO40036). Multicentre, randomised, double‐blind, double‐dummy, parallel group, 104‐week study to compare the effect of the salmeterol/fluticasone propionate combination product (SERETIDE*) 50/500mcg delivered twice daily via the DISKUS*/ACCUHALER* inhaler with tiotropium bromide 18 mcg delivered once daily via the HandiHaler inhalation device on the rate of health care utilisation exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). http://ctr.gsk.co.uk (accessed 8th April 2008).
-
- Seemungal T, Stockley R, Calverley P, Hagan G, Wedzicha JA. Investigating new standards for prophylaxis in reduction of exacerbations ‐ The INSPIRE study methodology. Journal of Chronic Obstructive Pulmonary Disease 2007;4(3):177‐83. - PubMed
-
- Wedzicha J, Stockley R, Seemungal T, Hagan G, Calverley P. The INSPIRE study: effect of salmeterol/fluticasone propionate versus tiotropium bromide on COPD exacerbations. Respirology 2007;12 Suppl 4:A112.
Lindberg 2007 {published data only}
-
- Lindberg A, Szalai Z, Pullerits T, Radeczky E. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology 2007;12(5):732‐9. - PubMed
-
- Lindberg A, Szalai Z, Pullertis T, Radeczky El. Budesonide/formoterol (B/F) has an onset of action that is similar to salbutamol and faster than salmeterol/fluticasone in patients with COPD. European Respiratory Journal 2006;28 Suppl 50:214s.
Schermer 2007 {published data only}
-
- Schermer TR, Albers JM, Verblackt HW, Costongs RJ, Westers P. Lower inhaled steroid requirement with a fluticasone/salmeterol combination in family practice patients with asthma or COPD. Family Practice 2007;24(2):181‐8.. - PubMed
Sethi 2006 {published data only}
-
- Sethi S, Grove L, Wrona C, Maloney J. Prevalence of bacterial colonization in COPD is not altered by fluticasone/salmeterol. Proceedings of the American Thoracic Society. 2006:A115.
Sutherland 2006 {published data only}
-
- Sutherland ER, Moss TA, Stevens AD, Pak J, Martin RJ. Modulation of sputum gene expression in COPD by fluticasone /salmeterol. European Respiratory Journal 2006;28 Suppl 50:662s.
Trofimenko 2006 {published data only}
-
- Trofimenko IN, Chernyak BA. The efficacy of salmeterol/fluticasone (SF) for 6 month's therapy at severe COPD patients. European Respiratory Journal 2006;28 Suppl 50:30s.
Zheng 2006 {published data only}
-
- Zheng J, Zhong N, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of fluticasone propionate 500 mg/salmeterol 50 mg combined via diskus/accuhaler in Chinese patients with chronic obstructive pulmonary disease (COPD). Chest 2006;130(4):182s.
-
- Zhong N, Zheng J, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of salmeterol 50µg/fluticasone propionate 500µg combined via accuhaler in Chinese patients with chronic obstructive pulmonary disease [Abstract]. Respirology 2006;11 Suppl 5:A150.
Additional references
Appleton 2006
Burge 2000
Calverley 2005
-
- Calverley P, Pauwels R, Lofdahl CG, Svensson K, Higenbottam T, Carlsson LG, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. - PubMed
Cazzola 2008
-
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31:416‐68. - PubMed
Celli 2008
-
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American Journal of Respiratory and Critical Care Medicine 2008;178(4):332‐8. - PubMed
Dahl 2001
-
- Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. American Journal of Resiratory Critical Care Medicine 2001;164(5):778–84. - PubMed
GOLD 2010
-
- Global Strategy for Diagnosis, Management, Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease. http://www.goldcopd.org 2010.
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jones 2005
-
- Jones PW. St George’s Respiratory Questionnaire: MCID. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2:75‐9. - PubMed
Karner 2011
-
- Karner C, Cates CJ. Long‐acting beta2‐agonist in addition to tiotropium versus either tiotropium or long‐acting beta2‐agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008989] - DOI
Karner 2011a
Karner 2011b
Karner 2012
Leidy 2003
-
- Leidy NK, Schmier JK, Jones MKC, et al. Evaluating symptoms in chronic obstructive pulmonary disease: validation of the Breathlessness, Cough and Sputum Scale. Respiratory Medicine 2003;97 Suppl A:59‐70. - PubMed
Mahler 1999
-
- Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115(4):957‐65. - PubMed
Miravitlles 2004
Miravitlles 2007
-
- Miravitlles M, Anzueto A, Legnani D, Forstmeier L, Fargel M. Patient's perception of exacerbations of COPD ‐ the PERCEIVE study. Respiratory Medicine 2007;101(3):453‐60. - PubMed
Nannini 2007a
Nannini 2010
NICE 2010
-
- National Collaborating Centre for Chronic Conditions. Managament of chronic obstructive pulmonary disease in primary and secondary care. http://www.nice.org.uk 2010;available at http://guidance.nice.org.uk/CG101/Guidance/pdf/English:1‐673.
Osman 1997
RevMan 5 [Computer program]
-
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Roisin 2000
-
- Rodriguez‐Roisin R. Toward a consensus definition of for COPD exacerbations. Chest 2000;117(5):398s‐401s. - PubMed
Singh 2009
-
- Singh S, Amin AV, Loke YK. Long‐term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta‐analysis. Archives of Internal Medicine 2009;169(3):219‐29. [PUBMED: 19204211] - PubMed
Singh 2010
Spencer 2011
-
- Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007033.pub3] - DOI
Suissa 2006
-
- Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2006;173(8):842‐6. - PubMed
Sutherland 2003
Visual Rx [Computer program]
-
- Cates CJ. Visual Rx 2.0. 2003.
Welsh 2011
-
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007891.pub2] - DOI
References to other published versions of this review
Nannini 2002
Nannini 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

