XBP1 depletion precedes ubiquitin aggregation and Golgi fragmentation in TDP-43 transgenic rats
- PMID: 22970712
- PMCID: PMC3534861
- DOI: 10.1111/jnc.12014
XBP1 depletion precedes ubiquitin aggregation and Golgi fragmentation in TDP-43 transgenic rats
Abstract
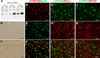
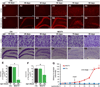
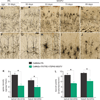
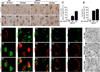
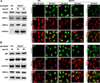
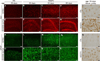
Protein inclusion is a prominent feature of neurodegenerative diseases including frontotemporal lobar degeneration (FTLD) that is characterized by the presence of ubiquitinated TDP-43 inclusion. Presence of protein inclusions indicates an interruption to protein degradation machinery or the overload of misfolded proteins. In response to the increase in misfolded proteins, cells usually initiate a mechanism called unfolded protein response (UPR) to reduce misfolded proteins in the lumen of endoplasmic reticules. Here, we examined the effects of mutant TDP-43 on the UPR in transgenic rats that express mutant human TDP-43 restrictedly in the neurons of the forebrain. Over-expression of mutant TDP-43 in rats caused prominent aggregation of ubiquitin and remarkable fragmentation of Golgi complexes prior to neuronal loss. While ubiquitin aggregates and Golgi fragments were accumulating, neurons expressing mutant TDP-43 failed to up-regulate chaperones residing in the endoplasmic reticules and failed to initiate the UPR. Prior to ubiquitin aggregation and Golgi fragmentation, neurons were depleted of X-box-binding protein 1 (XBP1), a key player of UPR machinery. Although it remains to determine how mutation of TDP-43 leads to the failure of the UPR, our data demonstrate that failure of the UPR is implicated in TDP-43 pathogenesis.
© 2012 The Authors Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Conflict of interest statement
Conflict of interest: The authors declare that no conflict of interest exists.
Figures
Similar articles
-
Entorhinal cortical neurons are the primary targets of FUS mislocalization and ubiquitin aggregation in FUS transgenic rats.Hum Mol Genet. 2012 Nov 1;21(21):4602-14. doi: 10.1093/hmg/dds299. Epub 2012 Jul 23. Hum Mol Genet. 2012. PMID: 22833456 Free PMC article.
-
A morphometric study of the spatial patterns of TDP-43 immunoreactive neuronal inclusions in frontotemporal lobar degeneration (FTLD) with progranulin (GRN) mutation.Histol Histopathol. 2011 Feb;26(2):185-90. doi: 10.14670/HH-26.185. Histol Histopathol. 2011. PMID: 21154232 Free PMC article.
-
TDP-43-induced death is associated with altered regulation of BIM and Bcl-xL and attenuated by caspase-mediated TDP-43 cleavage.J Biol Chem. 2011 Apr 15;286(15):13171-83. doi: 10.1074/jbc.M110.197483. Epub 2011 Feb 21. J Biol Chem. 2011. PMID: 21339291 Free PMC article.
-
[TDP-43 proteinopathies - from frontotemporal lobar degeneration to inclusion body myositis].Neurol Neurochir Pol. 2012 Jul-Aug;46(4):384-91. doi: 10.5114/ninp.2012.30271. Neurol Neurochir Pol. 2012. PMID: 23023438 Review. Polish.
-
Review: transactive response DNA-binding protein 43 (TDP-43): mechanisms of neurodegeneration.Neuropathol Appl Neurobiol. 2010 Apr;36(2):97-112. doi: 10.1111/j.1365-2990.2010.01060.x. Epub 2010 Feb 19. Neuropathol Appl Neurobiol. 2010. PMID: 20202122 Free PMC article. Review.
Cited by
-
Golgi fragmentation in amyotrophic lateral sclerosis, an overview of possible triggers and consequences.Front Neurosci. 2015 Oct 27;9:400. doi: 10.3389/fnins.2015.00400. eCollection 2015. Front Neurosci. 2015. PMID: 26578862 Free PMC article. Review.
-
Pathomechanisms of TDP-43 in neurodegeneration.J Neurochem. 2018 Feb 27:10.1111/jnc.14327. doi: 10.1111/jnc.14327. Online ahead of print. J Neurochem. 2018. PMID: 29486049 Free PMC article. Review.
-
ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43.Nat Commun. 2014 Jun 3;5:3996. doi: 10.1038/ncomms4996. Nat Commun. 2014. PMID: 24893131 Free PMC article.
-
Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery.PLoS One. 2014 Sep 8;9(9):e106837. doi: 10.1371/journal.pone.0106837. eCollection 2014. PLoS One. 2014. PMID: 25198176 Free PMC article.
-
PERK-opathies: An Endoplasmic Reticulum Stress Mechanism Underlying Neurodegeneration.Curr Alzheimer Res. 2016;13(2):150-63. doi: 10.2174/1567205013666151218145431. Curr Alzheimer Res. 2016. PMID: 26679859 Free PMC article. Review.
References
-
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

