Identifying viral parameters from in vitro cell cultures
- PMID: 22969758
- PMCID: PMC3432869
- DOI: 10.3389/fmicb.2012.00319
Identifying viral parameters from in vitro cell cultures
Abstract
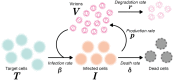
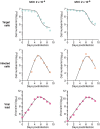
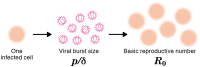
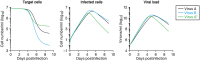
Current in vitro cell culture studies of viral replication deliver detailed time courses of several virological variables, like the amount of virions and the number of target cells, measured over several days of the experiment. Each of these time points solely provides a snap-shot of the virus infection kinetics and is brought about by the complex interplay of target cell infection, and viral production and cell death. It remains a challenge to interpret these data quantitatively and to reveal the kinetics of these underlying processes to understand how the viral infection depends on these kinetic properties. In order to decompose the kinetics of virus infection, we introduce a method to "quantitatively" describe the virus infection in in vitro cell cultures, and discuss the potential of the mathematical based analyses for experimental virology.
Keywords: in vitro experiment; mathematical modeling; quantification; virus infection.
Figures
Similar articles
-
Quantification system for the viral dynamics of a highly pathogenic simian/human immunodeficiency virus based on an in vitro experiment and a mathematical model.Retrovirology. 2012 Feb 25;9:18. doi: 10.1186/1742-4690-9-18. Retrovirology. 2012. PMID: 22364292 Free PMC article.
-
Molecular biological assessment methods and understanding the course of the HIV infection.APMIS Suppl. 2003;(114):1-37. APMIS Suppl. 2003. PMID: 14626050 Review.
-
Modelling Degradation and Replication Kinetics of the Zika Virus In Vitro Infection.Viruses. 2020 May 15;12(5):547. doi: 10.3390/v12050547. Viruses. 2020. PMID: 32429277 Free PMC article.
-
Production of Defective Interfering Particles of Influenza A Virus in Parallel Continuous Cultures at Two Residence Times-Insights From qPCR Measurements and Viral Dynamics Modeling.Front Bioeng Biotechnol. 2019 Oct 18;7:275. doi: 10.3389/fbioe.2019.00275. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31681751 Free PMC article.
-
Mathematical Analysis of Viral Replication Dynamics and Antiviral Treatment Strategies: From Basic Models to Age-Based Multi-Scale Modeling.Front Microbiol. 2018 Jul 11;9:1546. doi: 10.3389/fmicb.2018.01546. eCollection 2018. Front Microbiol. 2018. PMID: 30050523 Free PMC article. Review.
Cited by
-
Cell-to-cell infection by HIV contributes over half of virus infection.Elife. 2015 Oct 6;4:e08150. doi: 10.7554/eLife.08150. Elife. 2015. PMID: 26441404 Free PMC article.
-
Quantifying the Antiviral Effect of IFN on HIV-1 Replication in Cell Culture.Sci Rep. 2015 Jun 29;5:11761. doi: 10.1038/srep11761. Sci Rep. 2015. PMID: 26119462 Free PMC article.
-
Quantification of viral infection dynamics in animal experiments.Front Microbiol. 2013 Sep 10;4:264. doi: 10.3389/fmicb.2013.00264. Front Microbiol. 2013. PMID: 24058361 Free PMC article. Review.
-
A quantitative model used to compare within-host SARS-CoV-2, MERS-CoV, and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2.PLoS Biol. 2021 Mar 22;19(3):e3001128. doi: 10.1371/journal.pbio.3001128. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33750978 Free PMC article.
-
CD8+ Cytotoxic-T-Lymphocyte Breadth Could Facilitate Early Immune Detection of Immunodeficiency Virus-Derived Epitopes with Limited Expression Levels.mSphere. 2019 Jan 9;4(1):e00381-18. doi: 10.1128/mSphere.00381-18. mSphere. 2019. PMID: 30626618 Free PMC article.
References
-
- Anderson R. M. (1991). The Kermack-McKendrick epidemic threshold theorem. Bull. Math. Biol. 53, 3–32 - PubMed
LinkOut - more resources
Full Text Sources

