Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial
- PMID: 22968888
- PMCID: PMC3697088
- DOI: 10.1001/2012.jama.10893
Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial
Abstract
Context: No consensus exists for adjusting inhaled corticosteroid therapy in patients with asthma. Approaches include adjustment at outpatient visits guided by physician assessment of asthma control (symptoms, rescue therapy, pulmonary function), based on exhaled nitric oxide, or on a day-to-day basis guided by symptoms.
Objective: To determine if adjustment of inhaled corticosteroid therapy based on exhaled nitric oxide or day-to-day symptoms is superior to guideline-informed, physician assessment-based adjustment in preventing treatment failure in adults with mild to moderate asthma.
Design, setting, and participants: A randomized, parallel, 3-group, placebo-controlled, multiply-blinded trial of 342 adults with mild to moderate asthma controlled by low-dose inhaled corticosteroid therapy (n = 114 assigned to physician assessment-based adjustment [101 completed], n = 115 to biomarker-based [exhaled nitric oxide] adjustment [92 completed], and n = 113 to symptom-based adjustment [97 completed]), the Best Adjustment Strategy for Asthma in the Long Term (BASALT) trial was conducted by the Asthma Clinical Research Network at 10 academic medical centers in the United States for 9 months between June 2007 and July 2010.
Interventions: For physician assessment-based adjustment and biomarker-based (exhaled nitric oxide) adjustment, the dose of inhaled corticosteroids was adjusted every 6 weeks; for symptom-based adjustment, inhaled corticosteroids were taken with each albuterol rescue use.
Main outcome measure: The primary outcome was time to treatment failure.
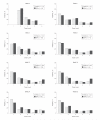
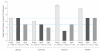
Results: There were no significant differences in time to treatment failure. The 9-month Kaplan-Meier failure rates were 22% (97.5% CI, 14%-33%; 24 events) for physician assessment-based adjustment, 20% (97.5% CI, 13%-30%; 21 events) for biomarker-based adjustment, and 15% (97.5% CI, 9%-25%; 16 events) for symptom-based adjustment. The hazard ratio for physician assessment-based adjustment vs biomarker-based adjustment was 1.2 (97.5% CI, 0.6-2.3). The hazard ratio for physician assessment-based adjustment vs symptom-based adjustment was 1.6 (97.5% CI, 0.8-3.3).
Conclusion: Among adults with mild to moderate persistent asthma controlled with low-dose inhaled corticosteroid therapy, the use of either biomarker-based or symptom-based adjustment of inhaled corticosteroids was not superior to physician assessment-based adjustment of inhaled corticosteroids in time to treatment failure.
Trial registration: clinicaltrials.gov Identifier: NCT00495157.
Figures


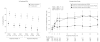
Comment in
-
Inhaled corticosteroid dose adjustment in mild persistent asthma.JAMA. 2012 Sep 12;308(10):1036-7. doi: 10.1001/2012.jama.11405. JAMA. 2012. PMID: 22968893 No abstract available.
-
Strategies for tailoring asthma treatment in adults.JAMA. 2013 Jan 9;309(2):135-6. doi: 10.1001/jama.2012.73379. JAMA. 2013. PMID: 23299596 No abstract available.
-
Strategies for tailoring asthma treatment in adults.JAMA. 2013 Jan 9;309(2):136. doi: 10.1001/jama.2012.73382. JAMA. 2013. PMID: 23299597 No abstract available.
-
Strategies for tailoring asthma treatment in adults--reply.JAMA. 2013 Jan 9;309(2):136-7. doi: 10.1001/jama.2012.73385. JAMA. 2013. PMID: 23299598 Free PMC article. No abstract available.
-
ACP Journal Club. Physician-, biomarker-, and symptom-based adjustment of inhaled corticosteroids for asthma had similar effects.Ann Intern Med. 2013 Jan 15;158(2):JC6. doi: 10.7326/0003-4819-158-2-201301150-02006. Ann Intern Med. 2013. PMID: 23318340 No abstract available.
Similar articles
-
Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults.Cochrane Database Syst Rev. 2009 Oct 7;(4):CD006340. doi: 10.1002/14651858.CD006340.pub3. Cochrane Database Syst Rev. 2009. PMID: 19821360 Review.
-
Composite type-2 biomarker strategy versus a symptom-risk-based algorithm to adjust corticosteroid dose in patients with severe asthma: a multicentre, single-blind, parallel group, randomised controlled trial.Lancet Respir Med. 2021 Jan;9(1):57-68. doi: 10.1016/S2213-2600(20)30397-0. Epub 2020 Sep 8. Lancet Respir Med. 2021. PMID: 32916135 Free PMC article. Clinical Trial.
-
Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD006340. doi: 10.1002/14651858.CD006340.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD006340. doi: 10.1002/14651858.CD006340.pub3 PMID: 18425949 Updated. Review.
-
The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial.Am J Respir Crit Care Med. 2007 Aug 1;176(3):231-7. doi: 10.1164/rccm.200610-1427OC. Epub 2007 May 11. Am J Respir Crit Care Med. 2007. PMID: 17496226 Clinical Trial.
-
Use of exhaled nitric oxide measurements to guide treatment in chronic asthma.N Engl J Med. 2005 May 26;352(21):2163-73. doi: 10.1056/NEJMoa043596. Epub 2005 May 24. N Engl J Med. 2005. PMID: 15914548 Clinical Trial.
Cited by
-
It is time to change the way we manage mild asthma: an update in GINA 2019.Respir Res. 2019 Aug 14;20(1):183. doi: 10.1186/s12931-019-1159-y. Respir Res. 2019. PMID: 31412856 Free PMC article.
-
Asthma Controller Medication Adherence, Risk of Exacerbation, and Use of Rescue Agents Among Texas Medicaid Patients with Persistent Asthma.J Manag Care Spec Pharm. 2015 Dec;21(12):1124-32. doi: 10.18553/jmcp.2015.21.12.1124. J Manag Care Spec Pharm. 2015. PMID: 26679962 Free PMC article.
-
2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group.J Allergy Clin Immunol. 2020 Dec;146(6):1217-1270. doi: 10.1016/j.jaci.2020.10.003. J Allergy Clin Immunol. 2020. PMID: 33280709 Free PMC article. Review.
-
Exhaled nitric oxide levels to guide treatment for children with asthma.Cochrane Database Syst Rev. 2016 Nov 9;11(11):CD011439. doi: 10.1002/14651858.CD011439.pub2. Cochrane Database Syst Rev. 2016. PMID: 27825189 Free PMC article. Review.
-
A Call for the United States to Accelerate the Implementation of Reliever Combination Inhaled Corticosteroid-Formoterol Inhalers in Asthma.Am J Respir Crit Care Med. 2023 Feb 15;207(4):390-405. doi: 10.1164/rccm.202209-1729PP. Am J Respir Crit Care Med. 2023. PMID: 36538711 Free PMC article. No abstract available.
References
-
- National Asthma Education and Prevention Program Expert Panel . Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute; Rockville, MD: 2007. NIH Publication 08-4051.
-
- Global Initiative for Asthma [Accessibility verified August 7, 2012];Global strategy for asthma management and prevention. http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asth....
-
- Sutherland ER. Nocturnal asthma. J Allergy Clin Immunol. 2005;116(6):1179–1186. - PubMed
-
- Smith AD, Cowan JO, Brassett KP, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352(21):2163–2173. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL074227/HL/NHLBI NIH HHS/United States
- UL1TR000071/TR/NCATS NIH HHS/United States
- U10 HL074204/HL/NHLBI NIH HHS/United States
- UL1 TR000071/TR/NCATS NIH HHS/United States
- M01 RR000073/RR/NCRR NIH HHS/United States
- U10 HL074231/HL/NHLBI NIH HHS/United States
- U10 HL074225/HL/NHLBI NIH HHS/United States
- U10 HL074073/HL/NHLBI NIH HHS/United States
- U10 HL074212/HL/NHLBI NIH HHS/United States
- U10 HL074206/HL/NHLBI NIH HHS/United States
- U10 HL074208/HL/NHLBI NIH HHS/United States
- U10 HL074218/HL/NHLBI NIH HHS/United States

