Macrophage migration inhibitory factor produced by the tumour stroma but not by tumour cells regulates angiogenesis in the B16-F10 melanoma model
- PMID: 22955855
- PMCID: PMC3493755
- DOI: 10.1038/bjc.2012.392
Macrophage migration inhibitory factor produced by the tumour stroma but not by tumour cells regulates angiogenesis in the B16-F10 melanoma model
Abstract
Background: Macrophage migration inhibitory factor (MIF) has been proposed as a link between inflammation and tumorigenesis. Despite its potentially broad influence in tumour biology and prevalent expression, the value of MIF as a therapeutic target in cancer remains unclear. We sought to validate MIF in tumour models by achieving a complete inhibition of its expression in tumour cells and in the tumour stroma.
Methods: We used MIF shRNA-transduced B16-F10 melanoma cells implanted in wild-type and MIF-/- C57Bl6 mice to investigate the effect of loss of MIF on tumour growth. Cytokine detection and immunohistochemistry (IHC) were used to evaluate tumours ex vivo.
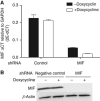
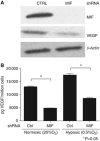
Results: Macrophage migration inhibitory factor shRNA inhibited expression of MIF protein by B16-F10 melanoma cells in vitro and in vivo. In vitro, the loss of MIF in this cell line resulted in a decreased response to hypoxia as indicated by reduced expression of VEGF. In vivo the growth of B16-F10 tumours was inhibited by an average of 47% in the MIF-/- mice compared with wild-type but was unaffected by loss of MIF expression by the tumour cells. Immunohistochemistry analysis revealed that microvessel density was decreased in tumours implanted in the MIF-/- mice. Profiling of serum cytokines showed a decrease in pro-angiogenic cytokines in MIF-/- mice.
Conclusion: We report that the absence of MIF in the host resulted in slower tumour growth, which was associated with reduced vascularity. While the major contribution of MIF appeared to be in the regulation of angiogenesis, tumour cell-derived MIF played a negligible role in this process.
Conflict of interest statement
All authors are or were Amgen Inc. employees and shareholders.
Figures
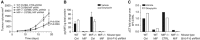


Similar articles
-
Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor.J Immunol. 2013 Mar 15;190(6):2984-93. doi: 10.4049/jimmunol.1201650. Epub 2013 Feb 6. J Immunol. 2013. PMID: 23390297 Free PMC article.
-
Interference of macrophage migration inhibitory factor expression in a mouse melanoma inhibits tumor establishment by up-regulating thrombospondin-1.Mol Cancer Res. 2007 Dec;5(12):1225-31. doi: 10.1158/1541-7786.MCR-07-0229. Mol Cancer Res. 2007. PMID: 18171979
-
Absence of macrophage migration inhibitory factor reduces proliferative retinopathy in a mouse model.Acta Diabetol. 2017 Apr;54(4):383-392. doi: 10.1007/s00592-016-0956-8. Epub 2017 Jan 9. Acta Diabetol. 2017. PMID: 28070752
-
Macrophage migration inhibitory factor (MIF): Its potential role in tumor growth and tumor-associated angiogenesis.Ann N Y Acad Sci. 2003 May;995:171-82. doi: 10.1111/j.1749-6632.2003.tb03220.x. Ann N Y Acad Sci. 2003. PMID: 12814949 Review.
-
Macrophage migration inhibitory factor involvement in breast cancer (Review).Int J Oncol. 2015 Nov;47(5):1627-33. doi: 10.3892/ijo.2015.3185. Epub 2015 Sep 24. Int J Oncol. 2015. PMID: 26412712 Free PMC article. Review.
Cited by
-
Vascular dysfunction and increased metastasis of B16F10 melanomas in Shb deficient mice as compared with their wild type counterparts.BMC Cancer. 2015 Apr 8;15:234. doi: 10.1186/s12885-015-1269-y. BMC Cancer. 2015. PMID: 25885274 Free PMC article.
-
Elevated serum levels of macrophage migration inhibitory factor are associated with progressive chronic cardiomyopathy in patients with Chagas disease.PLoS One. 2013;8(2):e57181. doi: 10.1371/journal.pone.0057181. Epub 2013 Feb 22. PLoS One. 2013. PMID: 23451183 Free PMC article.
-
Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets.Oncol Lett. 2016 Oct;12(4):2247-2253. doi: 10.3892/ol.2016.4929. Epub 2016 Aug 2. Oncol Lett. 2016. PMID: 27698786 Free PMC article.
-
Macrophage-tumor cell fusions from peripheral blood of melanoma patients.PLoS One. 2015 Aug 12;10(8):e0134320. doi: 10.1371/journal.pone.0134320. eCollection 2015. PLoS One. 2015. PMID: 26267609 Free PMC article.
-
Oral squamous carcinoma cells promote macrophage polarization in an MIF-dependent manner.QJM. 2018 Nov 1;111(11):769-778. doi: 10.1093/qjmed/hcy163. QJM. 2018. PMID: 30016493 Free PMC article.
References
-
- Abe R, Peng T, Sailors J, Bucala R, Metz CN (2001) Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol 166: 747–753 - PubMed
-
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E (2006) The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 25: 387–408 - PubMed
-
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13: 587–596 - PubMed
-
- Bifulco C, McDaniel K, Leng L, Bucala R (2008) Tumor growth-promoting properties of macrophage migration inhibitory factor. Cur Pharm Des 14: 3790–3801 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

