Characterization of the conversion between CD133+ and CD133- cells in colon cancer SW620 cell line
- PMID: 22954703
- PMCID: PMC3542230
- DOI: 10.4161/cbt.22000
Characterization of the conversion between CD133+ and CD133- cells in colon cancer SW620 cell line
Abstract
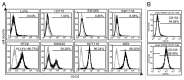
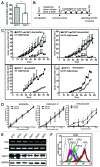
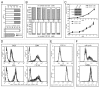
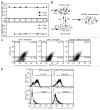
The state of cancer stem cells (CSC) under reversible fluctuations, which has been revealed in breast cancer cells most recently, suggests that subpopulations with distinct phenotypes and functions within cancer cells can undergo inter-conversion. To investigate the possibility in colon cancer cells, we employed CD133 as the CSC marker, and characterized CD133 expression pattern and the biological features of the CD133 (+) and CD133 (-) subsets. Flow cytometry revealed that CD133 was bimodally expressed in SW620 cells among eight colon cancer cell lines. The CD133 (+) clonal SW620 cells displayed a differential gene expression profile, higher cellular reactive oxygen species (ROS), enhanced tumorigenesis and resistance to 5-fluorouracil. The conversion in term of the CD133 phenotype of the sorted cells was observed in vitro and in vivo. The fraction of the CD133 (+) cells decreased from 99% to 80% in the sorted CD133 (+) population while rising from 5 to 10% in the sorted CD133 (-) population during the first 20-day cultivation and then stayed almost unchanged. A fraction (about 20%) of the CD133 (+) clonal cells lost their CD133 marker while about 10% of the CD133 (-) clonal cells acquired the CD133 marker. 5-Azacytidine enhanced the fraction of the CD133 (+) cells in both of the CD133 (+) and CD133 (-) clonal cells. Our data demonstrate that CD133 expression is dynamic and reversible, and reveal the inter-conversion between the CD133 (+) and the CD133 (-) SW620 cells, suggesting that the CD133 phenotype of SW620 cell population is retained by the conversion between the two cell subsets.
Figures

Similar articles
-
CD133 and CD133-regulated nucleophosmin linked to 5-fluorouracil susceptibility in human colon cancer cell line SW620.Electrophoresis. 2014 Feb;35(4):522-32. doi: 10.1002/elps.201300364. Epub 2013 Dec 16. Electrophoresis. 2014. PMID: 24339132
-
Characteristics of CD133(+) human colon cancer SW620 cells.Cell Transplant. 2010;19(6):857-64. doi: 10.3727/096368910X508988. Epub 2010 Jun 29. Cell Transplant. 2010. PMID: 20587144
-
Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer.Oncol Rep. 2012 Oct;28(4):1301-8. doi: 10.3892/or.2012.1951. Epub 2012 Aug 6. Oncol Rep. 2012. PMID: 22895640 Free PMC article.
-
Research progression of CD133 as a marker of cancer stem cells.Chin J Cancer. 2010 Mar;29(3):243-7. doi: 10.5732/cjc.009.10587. Chin J Cancer. 2010. PMID: 20193104 Review.
-
Melanoma tumor cell heterogeneity: a molecular approach to study subpopulations expressing the embryonic morphogen nodal.Semin Oncol. 2014 Apr;41(2):259-266. doi: 10.1053/j.seminoncol.2014.02.001. Epub 2014 Feb 7. Semin Oncol. 2014. PMID: 24787297 Free PMC article. Review.
Cited by
-
Electrical Impedance Spectroscopy for Monitoring Chemoresistance of Cancer Cells.Micromachines (Basel). 2020 Aug 31;11(9):832. doi: 10.3390/mi11090832. Micromachines (Basel). 2020. PMID: 32878225 Free PMC article. Review.
-
Cancer stem cells in colorectal cancer from pathogenesis to therapy: controversies and perspectives.World J Gastroenterol. 2014 Jan 28;20(4):923-42. doi: 10.3748/wjg.v20.i4.923. World J Gastroenterol. 2014. PMID: 24574766 Free PMC article. Review.
-
CD133: a stem cell biomarker and beyond.Exp Hematol Oncol. 2013 Jul 1;2(1):17. doi: 10.1186/2162-3619-2-17. Exp Hematol Oncol. 2013. PMID: 23815814 Free PMC article.
-
Statistical inference of the rates of cell proliferation and phenotypic switching in cancer.J Theor Biol. 2023 Jul 7;568:111497. doi: 10.1016/j.jtbi.2023.111497. Epub 2023 Apr 21. J Theor Biol. 2023. PMID: 37087049 Free PMC article.
-
An Fc-Optimized CD133 Antibody for Induction of Natural Killer Cell Reactivity against Colorectal Cancer.Cancers (Basel). 2019 Jun 7;11(6):789. doi: 10.3390/cancers11060789. Cancers (Basel). 2019. PMID: 31181683 Free PMC article.
References
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
