Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers
- PMID: 22952686
- PMCID: PMC3430690
- DOI: 10.1371/journal.pone.0043472
Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers
Abstract
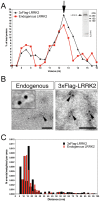
Leucine-rich repeat kinase 1 and 2 (LRRK1 and LRRK2) are large multidomain proteins containing kinase, GTPase and multiple protein-protein interaction domains, but only mutations in LRRK2 are linked to familial Parkinson's disease (PD). Independent studies suggest that LRRK2 exists in the cell as a complex compatible with the size of a dimer. However, whether this complex is truly a homodimer or a heterologous complex formed by monomeric LRRK2 with other proteins has not been definitively proven due to the limitations in obtaining highly pure proteins suitable for structural characterization. Here, we used stable expression of LRRK1 and LRRK2 in HEK293T cell lines to produce recombinant LRRK1 and LRRK2 proteins of greater than 90% purity. Both purified LRRKs are folded, with a predominantly alpha-helical secondary structure and are capable of binding GTP with similar affinity. Furthermore, recombinant LRRK2 exhibits robust autophosphorylation activity, phosphorylation of model peptides in vitro and ATP binding. In contrast, LRRK1 does not display significant autophosphorylation activity and fails to phosphorylate LRRK2 model substrates, although it does bind ATP. Using these biochemically validated proteins, we show that LRRK1 and LRRK2 are capable of forming homodimers as shown by single-particle transmission electron microscopy and immunogold labeling. These LRRK dimers display an elongated conformation with a mean particle size of 145 Å and 175 Å respectively, which is disrupted by addition of 6M guanidinium chloride. Immunogold staining revealed double-labeled particles also in the pathological LRRK2 mutant G2019S and artificial mutants disrupting GTPase and kinase activities, suggesting that point mutations do not hinder the dimeric conformation. Overall, our findings indicate for the first time that purified and active LRRK1 and LRRK2 can form dimers in their full-length conformation.
Conflict of interest statement
Figures
Similar articles
-
Cryo-EM analysis of homodimeric full-length LRRK2 and LRRK1 protein complexes.Sci Rep. 2017 Aug 17;7(1):8667. doi: 10.1038/s41598-017-09126-z. Sci Rep. 2017. PMID: 28819229 Free PMC article.
-
Deciphering the LRRK code: LRRK1 and LRRK2 phosphorylate distinct Rab proteins and are regulated by diverse mechanisms.Biochem J. 2021 Feb 12;478(3):553-578. doi: 10.1042/BCJ20200937. Biochem J. 2021. PMID: 33459343 Free PMC article.
-
Human leucine-rich repeat kinase 1 and 2: intersecting or unrelated functions?Biochem Soc Trans. 2012 Oct;40(5):1095-101. doi: 10.1042/BST20120123. Biochem Soc Trans. 2012. PMID: 22988872 Review.
-
PKC isoforms activate LRRK1 kinase by phosphorylating conserved residues (Ser1064, Ser1074 and Thr1075) within the CORB GTPase domain.Biochem J. 2022 Sep 30;479(18):1941-1965. doi: 10.1042/BCJ20220308. Biochem J. 2022. PMID: 36040231 Free PMC article.
-
Leucine-rich repeat kinase 2 (LRRK2): a key player in the pathogenesis of Parkinson's disease.J Neurosci Res. 2009 May 1;87(6):1283-95. doi: 10.1002/jnr.21949. J Neurosci Res. 2009. PMID: 19025767 Free PMC article. Review.
Cited by
-
A feed-forward pathway drives LRRK2 kinase membrane recruitment and activation.Elife. 2022 Sep 23;11:e79771. doi: 10.7554/eLife.79771. Elife. 2022. PMID: 36149401 Free PMC article.
-
GTP binding and intramolecular regulation by the ROC domain of Death Associated Protein Kinase 1.Sci Rep. 2012;2:695. doi: 10.1038/srep00695. Epub 2012 Sep 26. Sci Rep. 2012. PMID: 23019516 Free PMC article.
-
Pathological Functions of LRRK2 in Parkinson's Disease.Cells. 2020 Nov 30;9(12):2565. doi: 10.3390/cells9122565. Cells. 2020. PMID: 33266247 Free PMC article. Review.
-
Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells.Cells. 2021 Apr 22;10(5):981. doi: 10.3390/cells10050981. Cells. 2021. PMID: 33922322 Free PMC article. Review.
-
Arsenite stress down-regulates phosphorylation and 14-3-3 binding of leucine-rich repeat kinase 2 (LRRK2), promoting self-association and cellular redistribution.J Biol Chem. 2014 Aug 1;289(31):21386-400. doi: 10.1074/jbc.M113.528463. Epub 2014 Jun 18. J Biol Chem. 2014. PMID: 24942733 Free PMC article.
References
-
- Lewis PA (2009) The function of ROCO proteins in health and disease. Biol Cell 101: 183–191. - PubMed
-
- Marin I, van Egmond WN, van Haastert PJ (2008) The Roco protein family: a functional perspective. The FASEB Journal 22: 3103–3110. - PubMed
-
- Bosgraaf L, Van Haastert PJ (2003) Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta 1643: 5–10. - PubMed
-
- Marin I (2006) The Parkinson disease gene LRRK2: evolutionary and structural insights. MolBiolEvol 23: 2423–2433. - PubMed
-
- Marin I (2008) Ancient origin of the Parkinson disease gene LRRK2. J Mol Evol 67: 41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

