Innate non-specific cell substratum adhesion
- PMID: 22952588
- PMCID: PMC3432024
- DOI: 10.1371/journal.pone.0042033
Innate non-specific cell substratum adhesion
Abstract
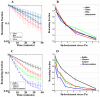
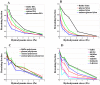
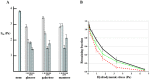
Adhesion of motile cells to solid surfaces is necessary to transmit forces required for propulsion. Unlike mammalian cells, Dictyostelium cells do not make integrin mediated focal adhesions. Nevertheless, they can move rapidly on both hydrophobic and hydrophilic surfaces. We have found that adhesion to such surfaces can be inhibited by addition of sugars or amino acids to the buffer. Treating whole cells with αlpha-mannosidase to cleave surface oligosaccharides also reduces adhesion. The results indicate that adhesion of these cells is mediated by van der Waals attraction of their surface glycoproteins to the underlying substratum. Since glycoproteins are prevalent components of the surface of most cells, innate adhesion may be a common cellular property that has been overlooked.
Conflict of interest statement
Figures

Similar articles
-
Locomotion and adhesion of polymorphonuclear leukocytes. Effects of the supporting substratum.Cell Biophys. 1982 Jun-Sep;4(2-3):133-41. doi: 10.1007/BF02918309. Cell Biophys. 1982. PMID: 6181880
-
SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand.J Cell Biochem. 1995 Feb;57(2):341-50. doi: 10.1002/jcb.240570218. J Cell Biochem. 1995. PMID: 7539008
-
Antibodies specific for gp40 inhibit cell-cell adhesion by cross-linking the protein on the surface of Dictyostelium purpureum.J Cell Biochem. 1993 Oct;53(2):85-97. doi: 10.1002/jcb.240530202. J Cell Biochem. 1993. PMID: 8227191
-
Cell adhesion in the life cycle of Dictyostelium.Experientia. 1995 Dec 18;51(12):1175-88. doi: 10.1007/BF01944735. Experientia. 1995. PMID: 8536805 Review.
-
Dimensions and dynamics in integrin function.Braz J Med Biol Res. 2003 Aug;36(8):959-66. doi: 10.1590/s0100-879x2003000800001. Epub 2003 Jul 23. Braz J Med Biol Res. 2003. PMID: 12886449 Review.
Cited by
-
Novel micropatterning technique reveals dependence of cell-substrate adhesion and migration of social amoebas on parental strain, development, and fluorescent markers.PLoS One. 2020 Jul 23;15(7):e0236171. doi: 10.1371/journal.pone.0236171. eCollection 2020. PLoS One. 2020. PMID: 32702047 Free PMC article.
-
Adhesion of Dictyostelium Amoebae to Surfaces: A Brief History of Attachments.Front Cell Dev Biol. 2022 May 27;10:910736. doi: 10.3389/fcell.2022.910736. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35721508 Free PMC article. Review.
-
Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes.Cell Mol Life Sci. 2014 Oct;71(19):3711-47. doi: 10.1007/s00018-014-1638-8. Epub 2014 May 21. Cell Mol Life Sci. 2014. PMID: 24846395 Free PMC article. Review.
-
Dictyostelium cells migrate similarly on surfaces of varying chemical composition.PLoS One. 2014 Feb 6;9(2):e87981. doi: 10.1371/journal.pone.0087981. eCollection 2014. PLoS One. 2014. PMID: 24516575 Free PMC article.
-
Cell-Substrate Patterns Driven by Curvature-Sensitive Actin Polymerization: Waves and Podosomes.Cells. 2020 Mar 23;9(3):782. doi: 10.3390/cells9030782. Cells. 2020. PMID: 32210185 Free PMC article.
References
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, et al. (2003) Cell migration: integrating signals from front to back. Science 302:1704–1709. Science 302: 1704–1709. - PubMed
-
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, et al. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453: 51–55. - PubMed
-
- Renkawitz J, Schumann K, Weber M, Lammermann T, Pflicke H, et al. (2009) Adaptive force transmission in amoeboid cell migration. Nat Cell Biol 11: 1438–1443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

