Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection
- PMID: 22952448
- PMCID: PMC3431332
- DOI: 10.1371/journal.ppat.1002898
Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection
Abstract
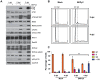
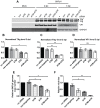
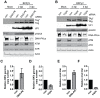
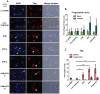
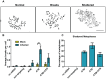
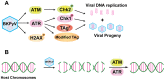
BK polyomavirus (BKPyV) is an emerging pathogen whose reactivation causes severe disease in transplant patients. Unfortunately, there is no specific anti-BKPyV treatment available, and host cell components that affect the infection outcome are not well characterized. In this report, we examined the relationship between BKPyV productive infection and the activation of the cellular DNA damage response (DDR) in natural host cells. Our results showed that both the ataxia-telangiectasia mutated (ATM)- and ATM and Rad-3-related (ATR)-mediated DDR were activated during BKPyV infection, accompanied by the accumulation of polyploid cells. We assessed the involvement of ATM and ATR during infection using small interfering RNA (siRNA) knockdowns. ATM knockdown did not significantly affect viral gene expression, but reduced BKPyV DNA replication and infectious progeny production. ATR knockdown had a slightly more dramatic effect on viral T antigen (TAg) and its modified forms, DNA replication, and progeny production. ATM and ATR double knockdown had an additive effect on DNA replication and resulted in a severe reduction in viral titer. While ATM mainly led to the activation of pChk2 and ATR was primarily responsible for the activation of pChk1, knockdown of all three major phosphatidylinositol 3-kinase-like kinases (ATM, ATR, and DNA-PKcs) did not abolish the activation of γH2AX during BKPyV infection. Finally, in the absence of ATM or ATR, BKPyV infection caused severe DNA damage and aberrant TAg staining patterns. These results indicate that induction of the DDR by BKPyV is critical for productive infection, and that one of the functions of the DDR is to minimize the DNA damage which is generated during BKPyV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


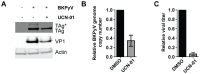
Similar articles
-
Host DNA damage response factors localize to merkel cell polyomavirus DNA replication sites to support efficient viral DNA replication.J Virol. 2014 Mar;88(6):3285-97. doi: 10.1128/JVI.03656-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390338 Free PMC article.
-
Viral DNA replication-dependent DNA damage response activation during BK polyomavirus infection.J Virol. 2015 May;89(9):5032-9. doi: 10.1128/JVI.03650-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694603 Free PMC article.
-
BK Polyomavirus Activates the DNA Damage Response To Prolong S Phase.J Virol. 2019 Jun 28;93(14):e00130-19. doi: 10.1128/JVI.00130-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043526 Free PMC article.
-
BK polyomavirus: emerging pathogen.Microbes Infect. 2012 Aug;14(9):672-83. doi: 10.1016/j.micinf.2012.02.002. Epub 2012 Feb 24. Microbes Infect. 2012. PMID: 22402031 Free PMC article. Review.
-
Human polyomavirus modulation of the host DNA damage response.Virus Genes. 2020 Apr;56(2):128-135. doi: 10.1007/s11262-020-01736-6. Epub 2020 Jan 29. Virus Genes. 2020. PMID: 31997082 Review.
Cited by
-
The DNA damage response promotes polyomavirus JC infection by nucleus to cytoplasm NF- kappaB activation.Virol J. 2017 Feb 15;14(1):31. doi: 10.1186/s12985-017-0707-7. Virol J. 2017. PMID: 28202068 Free PMC article.
-
Host DNA damage response factors localize to merkel cell polyomavirus DNA replication sites to support efficient viral DNA replication.J Virol. 2014 Mar;88(6):3285-97. doi: 10.1128/JVI.03656-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390338 Free PMC article.
-
Rad17 recruits the MRE11-RAD50-NBS1 complex to regulate the cellular response to DNA double-strand breaks.EMBO J. 2014 Apr 16;33(8):862-77. doi: 10.1002/embj.201386064. Epub 2014 Feb 16. EMBO J. 2014. PMID: 24534091 Free PMC article.
-
Polyomavirus interaction with the DNA damage response.Virol Sin. 2015 Apr;30(2):122-9. doi: 10.1007/s12250-015-3583-6. Epub 2015 Apr 20. Virol Sin. 2015. PMID: 25910481 Free PMC article. Review.
-
Analysis of viruses present in urine from patients with interstitial cystitis.Virus Genes. 2020 Aug;56(4):430-438. doi: 10.1007/s11262-020-01767-z. Epub 2020 May 23. Virus Genes. 2020. PMID: 32447589 Free PMC article.
References
-
- Gardner SD, Field AM, Coleman DV, Hulme B (1971) New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1: 1253–1257. - PubMed
-
- Imperiale MJ, Major EO (2007) Polyomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Fifth ed. Philadelphia, PA: Lippincott Williams & Wilkins. pp. 2263–2298.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

