The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth
- PMID: 22946456
- PMCID: PMC3572557
- DOI: 10.1111/j.1476-5381.2012.02195.x
The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth
Abstract
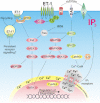
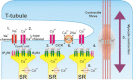
Endothelin-1 (ET-1) is a critical autocrine and paracrine regulator of cardiac physiology and pathology. Produced locally within the myocardium in response to diverse mechanical and neurohormonal stimuli, ET-1 acutely modulates cardiac contractility. During pathological cardiovascular conditions such as ischaemia, left ventricular hypertrophy and heart failure, myocyte expression and activity of the entire ET-1 system is enhanced, allowing the peptide to both initiate and maintain maladaptive cellular responses. Both the acute and chronic effects of ET-1 are dependent on the activation of intracellular signalling pathways, regulated by the inositol-trisphosphate and diacylglycerol produced upon activation of the ET(A) receptor. Subsequent stimulation of protein kinases C and D, calmodulin-dependent kinase II, calcineurin and MAPKs modifies the systolic calcium transient, myofibril function and the activity of transcription factors that coordinate cellular remodelling. The precise nature of the cellular response to ET-1 is governed by the timing, localization and context of such signals, allowing the peptide to regulate both cardiomyocyte physiology and instigate disease.
Linked articles: This article is part of a themed section on Endothelin. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2013.168.issue-1.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

Similar articles
-
Themed section: endothelin.Br J Pharmacol. 2013 Jan;168(2):279-82. doi: 10.1111/bph.12022. Br J Pharmacol. 2013. PMID: 23278331 Free PMC article.
-
Endothelin signalling in the cardiac myocyte and its pathophysiological relevance.Curr Vasc Pharmacol. 2005 Oct;3(4):343-51. doi: 10.2174/157016105774329390. Curr Vasc Pharmacol. 2005. PMID: 16248777 Review.
-
Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy.J Biol Chem. 1996 Feb 9;271(6):3221-8. doi: 10.1074/jbc.271.6.3221. J Biol Chem. 1996. PMID: 8621724
-
The role of endothelins and their receptors in heart failure.Pharmacol Res. 2001 Feb;43(2):111-26. doi: 10.1006/phrs.2000.0758. Pharmacol Res. 2001. PMID: 11243712 Review.
-
Use of A-192621 to provide evidence for involvement of endothelin ET(B)-receptors in endothelin-1-mediated cardiomyocyte hypertrophy.Eur J Pharmacol. 2001 Apr 13;417(3):157-68. doi: 10.1016/s0014-2999(01)00905-0. Eur J Pharmacol. 2001. PMID: 11334846
Cited by
-
Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy.Cardiovasc Res. 2017 Sep 1;113(11):1329-1337. doi: 10.1093/cvr/cvx103. Cardiovasc Res. 2017. PMID: 28575410 Free PMC article.
-
Autocrine Signaling in Cardiac Remodeling: A Rich Source of Therapeutic Targets.J Am Heart Assoc. 2021 Feb 2;10(3):e019169. doi: 10.1161/JAHA.120.019169. Epub 2021 Jan 20. J Am Heart Assoc. 2021. PMID: 33470124 Free PMC article. Review.
-
Cyclic nucleotide permeability through unopposed connexin hemichannels.Front Pharmacol. 2013 Jun 6;4:75. doi: 10.3389/fphar.2013.00075. eCollection 2013. Front Pharmacol. 2013. PMID: 23760880 Free PMC article.
-
Transcriptome profiling of 3D co-cultured cardiomyocytes and endothelial cells under oxidative stress using a photocrosslinkable hydrogel system.Acta Biomater. 2017 Aug;58:337-348. doi: 10.1016/j.actbio.2017.06.031. Epub 2017 Jun 23. Acta Biomater. 2017. PMID: 28648749 Free PMC article.
-
Vascularization of PEGylated fibrin hydrogels increases the proliferation of human iPSC-cardiomyocytes.J Biomed Mater Res A. 2024 Apr;112(4):625-634. doi: 10.1002/jbm.a.37662. Epub 2023 Dec 28. J Biomed Mater Res A. 2024. PMID: 38155509
References
-
- Allen BG, Phuong LL, Farhat H, Chevalier D. Both endothelin-A and endothelin-B receptors are present on adult rat cardiac ventricular myocytes. Can J Physiol Pharmacol. 2003;81:95–104. - PubMed
-
- Alvarez BV, Pérez NG, Ennis IL, Camilión de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca(2+) transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res. 1999;85:716–722. - PubMed
-
- Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. - PubMed
-
- Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther. 2005;106:39–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

