Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner
- PMID: 22942289
- PMCID: PMC3481266
- DOI: 10.1074/jbc.M112.393637
Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner
Abstract
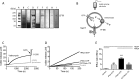
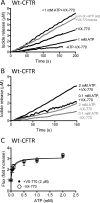
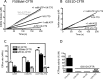
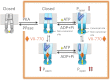
The cystic fibrosis transmembrane conductance regulator (CFTR) acts as a channel on the apical membrane of epithelia. Disease-causing mutations in the cystic fibrosis gene can lead to CFTR protein misfolding as in the case of the F508del mutation and/or channel dysfunction. Recently, a small molecule, VX-770 (ivacaftor), has shown efficacy in restoring lung function in patients bearing the G551D mutation, and this has been linked to repair of its channel gating defect. However, these studies did not reveal the mechanism of action of VX-770 in detail. Normally, CFTR channel activity is regulated by phosphorylation, ATP binding, and hydrolysis. Hence, it has been hypothesized that VX-770 modifies one or more of these metabolic events. In this study, we examined VX-770 activity using a reconstitution system for purified CFTR protein, a system that enables control of known regulatory factors. We studied the consequences of VX-770 interaction with CFTR incorporated in planar lipid bilayers and in proteoliposomes, using a novel flux-based assay. We found that purified and phosphorylated CFTR was potentiated in the presence of Mg-ATP, suggesting that VX-770 bound directly to the CFTR protein, rather than associated kinases or phosphatases. Interestingly, we also found that VX-770 enhanced the channel activity of purified and mutant CFTR in the nominal absence of Mg-ATP. These findings suggest that VX-770 can cause CFTR channel opening through a nonconventional ATP-independent mechanism. This work sets the stage for future studies of the structural properties that mediate CFTR gating using VX-770 as a probe.
Figures



Similar articles
-
Synergistic Potentiation of Cystic Fibrosis Transmembrane Conductance Regulator Gating by Two Chemically Distinct Potentiators, Ivacaftor (VX-770) and 5-Nitro-2-(3-Phenylpropylamino) Benzoate.Mol Pharmacol. 2016 Sep;90(3):275-85. doi: 10.1124/mol.116.104570. Epub 2016 Jul 13. Mol Pharmacol. 2016. PMID: 27413118 Free PMC article.
-
A small molecule CFTR potentiator restores ATP-dependent channel gating to the cystic fibrosis mutant G551D-CFTR.Br J Pharmacol. 2022 Apr;179(7):1319-1337. doi: 10.1111/bph.15709. Epub 2022 Jan 21. Br J Pharmacol. 2022. PMID: 34644413 Free PMC article.
-
Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle.Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):4404-9. doi: 10.1073/pnas.1215982110. Epub 2013 Feb 25. Proc Natl Acad Sci U S A. 2013. PMID: 23440202 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
-
The gating of the CFTR channel.Cell Mol Life Sci. 2017 Jan;74(1):85-92. doi: 10.1007/s00018-016-2390-z. Epub 2016 Oct 1. Cell Mol Life Sci. 2017. PMID: 27696113 Free PMC article. Review.
Cited by
-
In Vitro Rescue of the Bile Acid Transport Function of ABCB11 Variants by CFTR Potentiators.Int J Mol Sci. 2022 Sep 15;23(18):10758. doi: 10.3390/ijms231810758. Int J Mol Sci. 2022. PMID: 36142670 Free PMC article.
-
Potentiation of the cystic fibrosis transmembrane conductance regulator by VX-770 involves stabilization of the pre-hydrolytic, O1 state.Br J Pharmacol. 2018 Oct;175(20):3990-4002. doi: 10.1111/bph.14475. Epub 2018 Sep 16. Br J Pharmacol. 2018. PMID: 30107029 Free PMC article.
-
Rapid therapeutic advances in CFTR modulator science.Pediatr Pulmonol. 2018 Nov;53(S3):S4-S11. doi: 10.1002/ppul.24157. Pediatr Pulmonol. 2018. PMID: 30289627 Free PMC article. Review.
-
Activity of lumacaftor is not conserved in zebrafish Cftr bearing the major cystic fibrosis-causing mutation.FASEB Bioadv. 2019 Sep 18;1(10):661-670. doi: 10.1096/fba.2019-00039. eCollection 2019 Oct. FASEB Bioadv. 2019. PMID: 32123813 Free PMC article.
-
Thymosin α-1 does not correct F508del-CFTR in cystic fibrosis airway epithelia.JCI Insight. 2018 Feb 8;3(3):e98699. doi: 10.1172/jci.insight.98699. eCollection 2018 Feb 8. JCI Insight. 2018. PMID: 29415893 Free PMC article.
References
-
- Proesmans M., Vermeulen F., De Boeck K. (2008) What's new in cystic fibrosis? From treating symptoms to correction of the basic defect. Eur. J. Pediatr. 167, 839–849 - PubMed
-
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834 - PubMed
-
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. (1992) Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358, 761–764 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

