Cellular cofactors of lentiviral integrase: from target validation to drug discovery
- PMID: 22928108
- PMCID: PMC3420096
- DOI: 10.1155/2012/863405
Cellular cofactors of lentiviral integrase: from target validation to drug discovery
Abstract
To accomplish their life cycle, lentiviruses make use of host proteins, the so-called cellular cofactors. Interactions between host cell and viral proteins during early stages of lentiviral infection provide attractive new antiviral targets. The insertion of lentiviral cDNA in a host cell chromosome is a step of no return in the replication cycle, after which the host cell becomes a permanent carrier of the viral genome and a producer of lentiviral progeny. Integration is carried out by integrase (IN), an enzyme playing also an important role during nuclear import. Plenty of cellular cofactors of HIV-1 IN have been proposed. To date, the lens epithelium-derived growth factor (LEDGF/p75) is the best studied cofactor of HIV-1 IN. Moreover, small molecules that block the LEDGF/p75-IN interaction have recently been developed for the treatment of HIV infection. The nuclear import factor transportin-SR2 (TRN-SR2) has been proposed as another interactor of HIV IN-mediating nuclear import of the virus. Using both proteins as examples, we will describe approaches to be taken to identify and validate novel cofactors as new antiviral targets. Finally, we will highlight recent advances in the design and the development of small-molecule inhibitors binding to the LEDGF/p75-binding pocket in IN (LEDGINs).
Figures

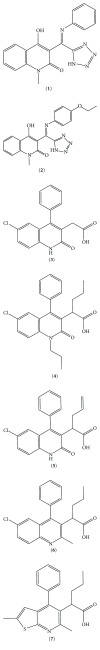
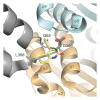
Similar articles
-
On the cell biology of HIV integration from basic research to development of novel antiviral drugs.Verh K Acad Geneeskd Belg. 2010;72(5-6):219-37. Verh K Acad Geneeskd Belg. 2010. PMID: 21409951 Review.
-
The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy.Virology. 2013 Jan 5;435(1):102-9. doi: 10.1016/j.virol.2012.09.033. Virology. 2013. PMID: 23217620 Review.
-
Structure-based virtual screening toward the discovery of novel inhibitors for impeding the protein-protein interaction between HIV-1 integrase and human lens epithelium-derived growth factor (LEDGF/p75).J Biomol Struct Dyn. 2018 Sep;36(12):3199-3217. doi: 10.1080/07391102.2017.1384400. Epub 2017 Oct 23. J Biomol Struct Dyn. 2018. PMID: 28948865
-
CPSF6-Dependent Targeting of Speckle-Associated Domains Distinguishes Primate from Nonprimate Lentiviral Integration.mBio. 2020 Sep 29;11(5):e02254-20. doi: 10.1128/mBio.02254-20. mBio. 2020. PMID: 32994325 Free PMC article.
-
LEDGINs, Inhibitors of the Interaction Between HIV-1 Integrase and LEDGF/p75, Are Potent Antivirals with a Potential to Cure HIV Infection.Adv Exp Med Biol. 2021;1322:97-114. doi: 10.1007/978-981-16-0267-2_4. Adv Exp Med Biol. 2021. PMID: 34258738
Cited by
-
Analysis of hepatic lentiviral vector transduction; implications for preclinical studies and clinical gene therapy protocols.bioRxiv [Preprint]. 2024 Aug 31:2024.08.20.608805. doi: 10.1101/2024.08.20.608805. bioRxiv. 2024. PMID: 39229157 Free PMC article. Preprint.
-
Inhibition of HIV Expression and Integration in Macrophages by Methylglyoxal-Bis-Guanylhydrazone.J Virol. 2015 Nov;89(22):11176-89. doi: 10.1128/JVI.01692-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223636 Free PMC article.
-
Identification of Vimentin as a Potential Therapeutic Target against HIV Infection.Viruses. 2016 Jun 15;8(6):98. doi: 10.3390/v8060098. Viruses. 2016. PMID: 27314381 Free PMC article.
-
HIV virions as nanoscopic test tubes for probing oligomerization of the integrase enzyme.ACS Nano. 2014 Apr 22;8(4):3531-45. doi: 10.1021/nn406615v. Epub 2014 Apr 2. ACS Nano. 2014. PMID: 24654558 Free PMC article.
-
Complex of HIV-1 Integrase with Cellular Ku Protein: Interaction Interface and Search for Inhibitors.Int J Mol Sci. 2022 Mar 8;23(6):2908. doi: 10.3390/ijms23062908. Int J Mol Sci. 2022. PMID: 35328329 Free PMC article.
References
-
- UNAIDS. Report on the global AIDS epidemic. Geneva, UNAIDS. 2010, http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008.
-
- Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annual Review of Pathology. 2011;6:223–248. - PubMed
-
- Nájera R, Delgado E, Pérez-Alvarez L, Thomson MM. Genetic recombination and its role in the development of the HIV-1 pandemic. AIDS. 2002;16(4):S3–S16. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
