Inhibitor of apoptosis protein (IAP) antagonists demonstrate divergent immunomodulatory properties in human immune subsets with implications for combination therapy
- PMID: 22923192
- PMCID: PMC11028923
- DOI: 10.1007/s00262-012-1342-1
Inhibitor of apoptosis protein (IAP) antagonists demonstrate divergent immunomodulatory properties in human immune subsets with implications for combination therapy
Abstract
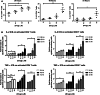
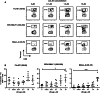
Inhibitor of apoptosis proteins (IAPs) are critical in regulating apoptosis resistance in cancer. Antagonists of IAPs, such as LCL161, are in clinical development and show promise as anti-cancer agents for solid and hematological cancers, with preliminary data suggesting they may act as immunomodulators. IAP antagonists hypersensitize tumor cells to TNF-α-mediated apoptosis, an effect that may work in synergy with that of cancer vaccines. This study aimed to further investigate the immunomodulatory properties of LCL161 on human immune subsets. T lymphocytes treated with LCL161 demonstrated significantly enhanced cytokine secretion upon activation, with little effect on CD4 and CD8 T-cell survival or proliferation. LCL161 treatment of peripheral blood mononuclear cells significantly enhanced priming of naïve T cells with synthetic peptides in vitro. Myeloid dendritic cells underwent phenotypic maturation upon IAP antagonism and demonstrated a reduced capacity to cross-present a tumor antigen-based vaccine. These effects are potentially mediated through an observed activation of the canonical and non-canonical NF-κB pathways, following IAP antagonism with a resulting upregulation of anti-apoptotic molecules. In conclusion, this study demonstrated the immunomodulatory properties of antagonists at physiologically relevant concentrations and indicates their combination with immunotherapy requires further investigation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
IAP inhibitors enhance co-stimulation to promote tumor immunity.J Exp Med. 2010 Sep 27;207(10):2195-206. doi: 10.1084/jem.20101123. Epub 2010 Sep 13. J Exp Med. 2010. PMID: 20837698 Free PMC article.
-
IAP antagonists induce anti-tumor immunity in multiple myeloma.Nat Med. 2016 Dec;22(12):1411-1420. doi: 10.1038/nm.4229. Epub 2016 Nov 14. Nat Med. 2016. PMID: 27841872 Free PMC article. Clinical Trial.
-
Blockade of inhibitors of apoptosis (IAPs) in combination with tumor-targeted delivery of tumor necrosis factor-α leads to synergistic antitumor activity.Cancer Gene Ther. 2013 Jan;20(1):46-56. doi: 10.1038/cgt.2012.83. Epub 2012 Nov 16. Cancer Gene Ther. 2013. PMID: 23154431 Free PMC article.
-
Anti-cancer IAP antagonists promote bone metastasis: a cautionary tale.J Bone Miner Metab. 2013 Sep;31(5):496-506. doi: 10.1007/s00774-013-0479-0. Epub 2013 Jun 6. J Bone Miner Metab. 2013. PMID: 23740289 Free PMC article. Review.
-
The IAP Protein Family, SMAC Mimetics and Cancer Treatment.Crit Rev Oncog. 2016;21(3-4):185-202. doi: 10.1615/CritRevOncog.2016017032. Crit Rev Oncog. 2016. PMID: 27915971 Review.
Cited by
-
ASTX660, an antagonist of cIAP1/2 and XIAP, increases antigen processing machinery and can enhance radiation-induced immunogenic cell death in preclinical models of head and neck cancer.Oncoimmunology. 2020 Jan 9;9(1):1710398. doi: 10.1080/2162402X.2019.1710398. eCollection 2020. Oncoimmunology. 2020. PMID: 32002309 Free PMC article.
-
Tumor microenvironment mimicking 3D models unveil the multifaceted effects of SMAC mimetics.iScience. 2023 Mar 11;26(4):106381. doi: 10.1016/j.isci.2023.106381. eCollection 2023 Apr 21. iScience. 2023. PMID: 37009211 Free PMC article.
-
IAP Antagonists Enhance Cytokine Production from Mouse and Human iNKT Cells.Cancer Immunol Res. 2018 Jan;6(1):25-35. doi: 10.1158/2326-6066.CIR-17-0490. Epub 2017 Nov 29. Cancer Immunol Res. 2018. PMID: 29187357 Free PMC article.
-
Targeting Upregulated cIAP2 in SOX10-Deficient Drug Tolerant Melanoma.Mol Cancer Ther. 2023 Sep 5;22(9):1087-1099. doi: 10.1158/1535-7163.MCT-23-0025. Mol Cancer Ther. 2023. PMID: 37343247 Free PMC article.
-
Astrocytes and the tumor microenvironment inflammatory state dictate the killing of glioblastoma cells by Smac mimetic compounds.Cell Death Dis. 2024 Aug 15;15(8):592. doi: 10.1038/s41419-024-06971-5. Cell Death Dis. 2024. PMID: 39147758 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

