Structural sweet spot for A1 adenosine receptor activation by truncated (N)-methanocarba nucleosides: receptor docking and potent anticonvulsant activity
- PMID: 22921089
- PMCID: PMC3463139
- DOI: 10.1021/jm300965a
Structural sweet spot for A1 adenosine receptor activation by truncated (N)-methanocarba nucleosides: receptor docking and potent anticonvulsant activity
Abstract
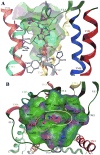
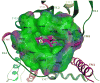
A(1) adenosine receptor (AR) agonists display antiischemic and antiepileptic neuroprotective activity, but peripheral cardiovascular side effects impeded their development. SAR study of N(6)-cycloalkylmethyl 4'-truncated (N)-methanocarba-adenosines identified 10 (MRS5474, N(6)-dicyclopropylmethyl, K(i) = 47.9 nM) as a moderately A(1)AR-selective full agonist. Two stereochemically defined N(6)-methynyl group substituents displayed narrow SAR; groups larger than cyclobutyl greatly reduced AR affinity, and those larger or smaller than cyclopropyl reduced A(1)AR selectivity. Nucleoside docking to A(1)AR homology model characterized distinct hydrophobic cyclopropyl subpockets, the larger "A" forming contacts with Thr270 (7.35), Tyr271 (7.36), Ile274 (7.39), and carbon chains of glutamates (EL2) and the smaller subpocket "B" forming contacts between TM6 and TM7. 10 suppressed minimal clonic seizures (6 Hz mouse model) without typical rotarod impairment of A(1)AR agonists. Truncated nucleosides, an appealing preclinical approach, have more druglike physicochemical properties than other A(1)AR agonists. Thus, we identified highly restricted regions for substitution around N(6) suitable for an A(1)AR agonist with anticonvulsant activity.
Figures

Similar articles
-
(N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists.J Med Chem. 2005 Mar 24;48(6):1745-58. doi: 10.1021/jm049580r. J Med Chem. 2005. PMID: 15771421 Free PMC article.
-
Design and in Vivo Characterization of A1 Adenosine Receptor Agonists in the Native Ribose and Conformationally Constrained (N)-Methanocarba Series.J Med Chem. 2019 Feb 14;62(3):1502-1522. doi: 10.1021/acs.jmedchem.8b01662. Epub 2019 Jan 3. J Med Chem. 2019. PMID: 30605331 Free PMC article.
-
Functionalized congeners of A3 adenosine receptor-selective nucleosides containing a bicyclo[3.1.0]hexane ring system.J Med Chem. 2009 Dec 10;52(23):7580-92. doi: 10.1021/jm900426g. J Med Chem. 2009. PMID: 19499950 Free PMC article.
-
Non-adenosine nucleoside inosine, guanosine and uridine as promising antiepileptic drugs: a summary of current literature.Mini Rev Med Chem. 2015;14(13):1033-42. doi: 10.2174/1389557514666141107120226. Mini Rev Med Chem. 2015. PMID: 25382017 Review.
-
Partial agonists for A(3) adenosine receptors.Curr Top Med Chem. 2004;4(8):855-62. doi: 10.2174/1568026043450989. Curr Top Med Chem. 2004. PMID: 15078216 Free PMC article. Review.
Cited by
-
Structural probing of off-target G protein-coupled receptor activities within a series of adenosine/adenine congeners.PLoS One. 2014 May 23;9(5):e97858. doi: 10.1371/journal.pone.0097858. eCollection 2014. PLoS One. 2014. PMID: 24859150 Free PMC article.
-
Selective modulation of epileptic tissue by an adenosine A3 receptor-activating drug.Br J Pharmacol. 2024 Dec;181(24):5041-5061. doi: 10.1111/bph.17319. Epub 2024 Sep 19. Br J Pharmacol. 2024. PMID: 39300608
-
Structure activity relationships of 5-HT2B and 5-HT2C serotonin receptor antagonists: N6, C2 and 5'-Modified (N)-methanocarba-adenosine derivatives.Eur J Med Chem. 2023 Nov 5;259:115691. doi: 10.1016/j.ejmech.2023.115691. Epub 2023 Jul 31. Eur J Med Chem. 2023. PMID: 37562117 Free PMC article.
-
Probing biased/partial agonism at the G protein-coupled A(2B) adenosine receptor.Biochem Pharmacol. 2014 Aug 1;90(3):297-306. doi: 10.1016/j.bcp.2014.05.008. Epub 2014 May 20. Biochem Pharmacol. 2014. PMID: 24853985 Free PMC article.
-
Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Mar 3;57:117-31. doi: 10.1016/j.pnpbp.2014.10.016. Epub 2014 Nov 7. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25445063 Free PMC article. Review.
References
-
- Giorgi I, Nieri P. Therapeutic potential of A1 adenosine receptor ligands: a survey of recent patent literature. Expert Opin Therap Patents. 2008;18:677–691.
-
- Zablocki JA, Wu L, Shryock J, Belardinelli L. Partial A1 adenosine receptor agonists from a molecular perspective and their potential use as chronic ventricular rate control agents during atrial fibrillation (AF) Curr Top Med Chem. 2004;4:839–854. - PubMed
- Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs. 2008;17:1901–1910. - PubMed
-
- Knutsen LJ, Lau J, Petersen H, Thomsen C, Weis JU, Shalmi M, Judge ME, Hansen AJ, Sheardown MJ. N-substituted adenosines as novel neuroprotective A1 agonists with diminished hypotensive effects. J Med Chem. 1999;42:3463–3477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

