Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins
- PMID: 22919642
- PMCID: PMC3417474
- DOI: 10.3389/fcimb.2012.00051
Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins
Abstract
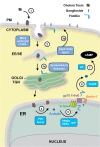
Some bacterial toxins and viruses have evolved the capacity to bind mammalian glycosphingolipids to gain access to the cell interior, where they can co-opt the endogenous mechanisms of cellular trafficking and protein translocation machinery to cause toxicity. Cholera toxin (CT) is one of the best-studied examples, and is the virulence factor responsible for massive secretory diarrhea seen in cholera. CT enters host cells by binding to monosialotetrahexosylganglioside (GM1 gangliosides) at the plasma membrane where it is transported retrograde through the trans-Golgi network (TGN) into the endoplasmic reticulum (ER). In the ER, a portion of CT, the CT-A1 polypeptide, is unfolded and then "retro-translocated" to the cytosol by hijacking components of the ER associated degradation pathway (ERAD) for misfolded proteins. CT-A1 rapidly refolds in the cytosol, thus avoiding degradation by the proteasome and inducing toxicity. Here, we highlight recent advances in our understanding of how the bacterial AB(5) toxins induce disease. We highlight the molecular mechanisms by which these toxins use glycosphingolipid to traffic within cells, with special attention to how the cell senses and sorts the lipid receptors. We also discuss several new studies that address the mechanisms of toxin unfolding in the ER and the mechanisms of CT A1-chain retro-translocation to the cytosol.
Keywords: ERAD; cholera toxin; membrane trafficking; retro-translocation.
Figures
Similar articles
-
Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum.Toxins (Basel). 2010 Mar;2(3):310-25. doi: 10.3390/toxins2030310. Epub 2010 Mar 5. Toxins (Basel). 2010. PMID: 22069586 Free PMC article. Review.
-
The intracellular voyage of cholera toxin: going retro.Trends Biochem Sci. 2003 Dec;28(12):639-45. doi: 10.1016/j.tibs.2003.10.002. Trends Biochem Sci. 2003. PMID: 14659695 Review.
-
Application of the biotin-labeled toxin mutant for affinity isolation of associated proteins in the mammalian cells.J Biosci Bioeng. 2018 May;125(5):497-504. doi: 10.1016/j.jbiosc.2017.12.002. Epub 2017 Dec 29. J Biosci Bioeng. 2018. PMID: 29291913
-
Raft trafficking of AB5 subunit bacterial toxins.Biochim Biophys Acta. 2005 Dec 30;1746(3):314-21. doi: 10.1016/j.bbamcr.2005.07.007. Epub 2005 Aug 15. Biochim Biophys Acta. 2005. PMID: 16153723 Review.
-
Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER.FEMS Microbiol Lett. 2007 Jan;266(2):129-37. doi: 10.1111/j.1574-6968.2006.00545.x. Epub 2006 Nov 29. FEMS Microbiol Lett. 2007. PMID: 17156122 Free PMC article. Review.
Cited by
-
Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells.Biomaterials. 2016 Feb;80:68-79. doi: 10.1016/j.biomaterials.2015.11.051. Epub 2015 Dec 2. Biomaterials. 2016. PMID: 26706477 Free PMC article.
-
A bacterial toxin and a nonenveloped virus hijack ER-to-cytosol membrane translocation pathways to cause disease.Crit Rev Biochem Mol Biol. 2015;50(6):477-88. doi: 10.3109/10409238.2015.1085826. Epub 2015 Sep 11. Crit Rev Biochem Mol Biol. 2015. PMID: 26362261 Free PMC article. Review.
-
Retrograde transport of protein toxins through the Golgi apparatus.Histochem Cell Biol. 2013 Sep;140(3):317-26. doi: 10.1007/s00418-013-1111-z. Epub 2013 Jun 14. Histochem Cell Biol. 2013. PMID: 23765164 Review.
-
Glycosylation of glycolipids in cancer: basis for development of novel therapeutic approaches.Front Oncol. 2013 Dec 19;3:306. doi: 10.3389/fonc.2013.00306. Front Oncol. 2013. PMID: 24392350 Free PMC article. Review.
-
Receptor-Mediated Sorting of Typhoid Toxin during Its Export from Salmonella Typhi-Infected Cells.Cell Host Microbe. 2016 Nov 9;20(5):682-689. doi: 10.1016/j.chom.2016.10.005. Cell Host Microbe. 2016. PMID: 27832592 Free PMC article.
References
-
- Amessou M., Fradagrada A., Falguieres T., Lord J. M., Smith D. C., Roberts L. M., Lamaze C., Johannes L. (2007). Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J. Cell Sci. 120, 1457–1468 10.1242/jcs.03436 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

