Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death
- PMID: 22918830
- PMCID: PMC3471765
- DOI: 10.1074/jbc.M112.394544
Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death
Abstract
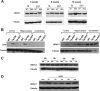
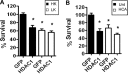
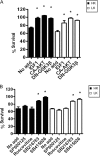
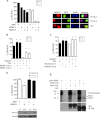
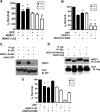
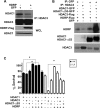
Both neuroprotective and neurotoxic roles have previously been described for histone deacetylase-1 (HDAC1). Here we report that HDAC1 expression is elevated in vulnerable brain regions of two mouse models of neurodegeneration, the R6/2 model of Huntington disease and the Ca(2+)/calmodulin-dependent protein kinase (CaMK)/p25 double-transgenic model of tauopathic degeneration, suggesting a role in promoting neuronal death. Indeed, elevating HDAC1 expression by ectopic expression promotes the death of otherwise healthy cerebellar granule neurons and cortical neurons in culture. The neurotoxic effect of HDAC1 requires interaction and cooperation with HDAC3, which has previously been shown to selectively induce the death of neurons. HDAC1-HDAC3 interaction is greatly elevated under conditions of neurodegeneration both in vitro and in vivo. Furthermore, the knockdown of HDAC3 suppresses HDAC1-induced neurotoxicity, and the knockdown of HDAC1 suppresses HDAC3 neurotoxicity. As described previously for HDAC3, the neurotoxic effect of HDAC1 is inhibited by treatment with IGF-1, the expression of Akt, or the inhibition of glycogen synthase kinase 3β (GSK3β). In addition to HDAC3, HDAC1 has been shown to interact with histone deacetylase-related protein (HDRP), a truncated form of HDAC9, whose expression is down-regulated during neuronal death. In contrast to HDAC3, the interaction between HDRP and HDAC1 protects neurons from death, an effect involving acquisition of the deacetylase activity of HDAC1 by HDRP. We find that elevated HDRP inhibits HDAC1-HDAC3 interaction and prevents the neurotoxic effect of either of these two proteins. Together, our results suggest that HDAC1 is a molecular switch between neuronal survival and death. Its interaction with HDRP promotes neuronal survival, whereas interaction with HDAC3 results in neuronal death.
Figures

Similar articles
-
Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3beta.J Neurosci. 2011 Feb 2;31(5):1746-51. doi: 10.1523/JNEUROSCI.5704-10.2011. J Neurosci. 2011. PMID: 21289184 Free PMC article.
-
Neuroprotection by histone deacetylase-related protein.Mol Cell Biol. 2006 May;26(9):3550-64. doi: 10.1128/MCB.26.9.3550-3564.2006. Mol Cell Biol. 2006. PMID: 16611996 Free PMC article.
-
Proteomic analysis identifies NPTX1 and HIP1R as potential targets of histone deacetylase-3-mediated neurodegeneration.Exp Biol Med (Maywood). 2018 Apr;243(7):627-638. doi: 10.1177/1535370218761149. Epub 2018 Feb 27. Exp Biol Med (Maywood). 2018. PMID: 29486577 Free PMC article.
-
HDAC1: an environmental sensor regulating endothelial function.Cardiovasc Res. 2022 Jun 29;118(8):1885-1903. doi: 10.1093/cvr/cvab198. Cardiovasc Res. 2022. PMID: 34264338 Free PMC article. Review.
-
Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective.Curr Neuropharmacol. 2022;20(1):158-178. doi: 10.2174/1570159X19666210609160017. Curr Neuropharmacol. 2022. PMID: 34151764 Free PMC article. Review.
Cited by
-
Mechanism of Action of 2-Aminobenzamide HDAC Inhibitors in Reversing Gene Silencing in Friedreich's Ataxia.Front Neurol. 2015 Mar 5;6:44. doi: 10.3389/fneur.2015.00044. eCollection 2015. Front Neurol. 2015. PMID: 25798128 Free PMC article.
-
The Role of Insulin-Like Growth Factors and Insulin-Like Growth Factor-Binding Proteins in the Nervous System.Biochem Insights. 2019 Apr 17;12:1178626419842176. doi: 10.1177/1178626419842176. eCollection 2019. Biochem Insights. 2019. PMID: 31024217 Free PMC article. Review.
-
Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin.J Lipid Res. 2014 Aug;55(8):1609-21. doi: 10.1194/jlr.R047837. Epub 2014 Mar 24. J Lipid Res. 2014. PMID: 24664998 Free PMC article. Review.
-
HDAC1 deregulation promotes neuronal loss and deficit of motor function in stroke pathogenesis.Sci Rep. 2021 Aug 11;11(1):16354. doi: 10.1038/s41598-021-95837-3. Sci Rep. 2021. PMID: 34381129 Free PMC article.
-
Subcellular Distribution of HDAC1 in Neurotoxic Conditions Is Dependent on Serine Phosphorylation.J Neurosci. 2017 Aug 2;37(31):7547-7559. doi: 10.1523/JNEUROSCI.3000-16.2017. Epub 2017 Jun 29. J Neurosci. 2017. PMID: 28663197 Free PMC article.
References
-
- Kazantsev A. G., Thompson L. M. (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 7, 854–868 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

