Long-term outcomes of pre-emptive valganciclovir compared with valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation
- PMID: 22917575
- PMCID: PMC3431418
- DOI: 10.1681/ASN.2012010100
Long-term outcomes of pre-emptive valganciclovir compared with valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation
Abstract
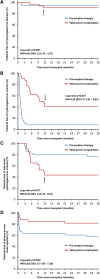
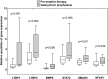
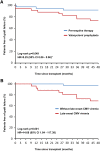
Prevention of cytomegalovirus (CMV) is essential in organ transplantation. The two main strategies are pre-emptive therapy, in which one screens for and treats asymptomatic CMV viremia, and universal antiviral prophylaxis. We compared these strategies and examined long-term outcomes in a randomized, open-label, single-center trial. We randomly assigned 70 renal transplant recipients (CMV-seropositive recipient or donor) to 3-month prophylaxis with valacyclovir (n=34) or pre-emptive valganciclovir for significant CMV viremia detected at predefined assessments through month 12 (n=36). Among the 55 patients who had a protocol biopsy specimen available at 3 years to allow assessment of the primary outcome, 9 (38%) of 24 patients in the prophylaxis group and 6 (19%) of 31 patients in the pre-emptive therapy group had moderate to severe interstitial fibrosis and tubular atrophy (odds ratio, 2.50; 95% confidence interval, 0.74-8.43; P=0.22). The prophylaxis group had significantly higher intrarenal mRNA expression of genes involved in fibrogenesis. The occurrence of CMV disease was similar in both groups, but pre-emptive therapy improved 4-year graft survival (92% versus 74%; P=0.049) as a result of worse outcomes in patients with late-onset CMV viremia. In conclusion, compared with valacyclovir prophylaxis, pre-emptive valganciclovir therapy may lead to less severe interstitial fibrosis and tubular atrophy and to significantly better graft survival.
Figures

Comment in
-
Randomized trial of pre-emptive or prophylactic valganciclovir therapy for prevention of cytomegalovirus infection in renal transplantation.J Am Soc Nephrol. 2012 Sep;23(9):1446-8. doi: 10.1681/ASN.2012070729. Epub 2012 Aug 9. J Am Soc Nephrol. 2012. PMID: 22878958 No abstract available.
-
Transplantation: Pre-emptive CMV therapy versus universal CMV prophylaxis in renal transplant recipients.Nat Rev Nephrol. 2012 Nov;8(11):614. doi: 10.1038/nrneph.2012.210. Epub 2012 Sep 18. Nat Rev Nephrol. 2012. PMID: 22986361 No abstract available.
Similar articles
-
Intragraft cytomegalovirus infection: a randomized trial of valacyclovir prophylaxis versus pre-emptive therapy in renal transplant recipients.Antivir Ther. 2010;15(1):23-30. doi: 10.3851/IMP1485. Antivir Ther. 2010. PMID: 20167988 Clinical Trial.
-
Randomized trial of valganciclovir versus valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation.Clin J Am Soc Nephrol. 2015 Feb 6;10(2):294-304. doi: 10.2215/CJN.07020714. Epub 2014 Nov 25. Clin J Am Soc Nephrol. 2015. PMID: 25424991 Free PMC article. Clinical Trial.
-
Less renal allograft fibrosis with valganciclovir prophylaxis for cytomegalovirus compared to high-dose valacyclovir: a parallel group, open-label, randomized controlled trial.BMC Infect Dis. 2018 Nov 15;18(1):573. doi: 10.1186/s12879-018-3493-y. BMC Infect Dis. 2018. PMID: 30442095 Free PMC article. Clinical Trial.
-
Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD003774. doi: 10.1002/14651858.CD003774.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2024 Apr 10;4:CD004667. doi: 10.1002/14651858.CD004667.pub6 Update in: Cochrane Database Syst Rev. 2024 May 3;5:CD003774. doi: 10.1002/14651858.CD003774.pub5 PMID: 23450543 Updated. Review.
-
The efficacy and cost-effectiveness of valacyclovir in cytomegalovirus prevention in solid organ transplantation.Expert Rev Pharmacoecon Outcomes Res. 2014 Dec;14(6):771-9. doi: 10.1586/14737167.2014.965157. Epub 2014 Sep 25. Expert Rev Pharmacoecon Outcomes Res. 2014. PMID: 25252996 Review.
Cited by
-
Usefulness of valacyclovir prophylaxis for cytomegalovirus infection after anti-thymocyte globulin as rejection therapy.Korean J Intern Med. 2019 Mar;34(2):375-382. doi: 10.3904/kjim.2017.040. Epub 2017 Dec 15. Korean J Intern Med. 2019. PMID: 29237252 Free PMC article.
-
Primary Cytomegalovirus Infection in Seronegative Kidney Transplant Patients Is Associated with Protracted Cold Ischemic Time of Seropositive Donor Organs.PLoS One. 2017 Jan 27;12(1):e0171035. doi: 10.1371/journal.pone.0171035. eCollection 2017. PLoS One. 2017. PMID: 28129395 Free PMC article.
-
A case report of CMV lymphadenitis in an adult kidney transplant recipient.Transplant Proc. 2015 Jan-Feb;47(1):141-5. doi: 10.1016/j.transproceed.2014.09.105. Transplant Proc. 2015. PMID: 25645793 Free PMC article.
-
Transplantation: Pre-emptive CMV therapy versus universal CMV prophylaxis in renal transplant recipients.Nat Rev Nephrol. 2012 Nov;8(11):614. doi: 10.1038/nrneph.2012.210. Epub 2012 Sep 18. Nat Rev Nephrol. 2012. PMID: 22986361 No abstract available.
-
Heterologous Cytomegalovirus and Allo-Reactivity by Shared T Cell Receptor Repertoire in Kidney Transplantation.Front Immunol. 2019 Oct 31;10:2549. doi: 10.3389/fimmu.2019.02549. eCollection 2019. Front Immunol. 2019. PMID: 31736968 Free PMC article. Clinical Trial.
References
-
- Fishman JA: Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614, 2007 - PubMed
-
- Reischig T: Cytomegalovirus-associated renal allograft rejection: New challenges for antiviral preventive strategies. Expert Rev Anti Infect Ther 8: 903–910, 2010 - PubMed
-
- Sagedal S, Nordal KP, Hartmann A, Sund S, Scott H, Degré M, Foss A, Leivestad T, Osnes K, Fauchald P, Rollag H: The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant 2: 850–856, 2002 - PubMed
-
- Reischig T, Jindra P, Hes O, Bouda M, Kormunda S, Treska V: Effect of cytomegalovirus viremia on subclinical rejection or interstitial fibrosis and tubular atrophy in protocol biopsy at 3 months in renal allograft recipients managed by preemptive therapy or antiviral prophylaxis. Transplantation 87: 436–444, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

