Regulatory T cells in radiotherapeutic responses
- PMID: 22912933
- PMCID: PMC3421147
- DOI: 10.3389/fonc.2012.00090
Regulatory T cells in radiotherapeutic responses
Abstract
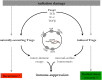
Radiation therapy (RT) can extend its influence in cancer therapy beyond what can be attributed to in-field cytotoxicity by modulating the immune system. While complex, these systemic effects can help tip the therapeutic balance in favor of treatment success or failure. Engagement of the immune system is generally through recognition of damage-associated molecules expressed or released as a result of tumor and normal tissue radiation damage. This system has evolved to discriminate pathological from physiological forms of cell death by signaling "danger." The multiple mechanisms that can be evoked include a shift toward a pro-inflammatory, pro-oxidant microenvironment that can promote maturation of dendritic cells and, in cancer treatment, the development of effector T cell responses to tumor-associated antigens. Control over these processes is exerted by regulatory T cells (Tregs), suppressor macrophages, and immunosuppressive cytokines that act in consort to maintain tolerance to self, limit tissue damage, and re-establish tissue homeostasis. Unfortunately, by the time RT for cancer is initiated the tumor-host relationship has already been sculpted in favor of tumor growth and against immune-mediated mechanisms for tumor regression. Reversing this situation is a major challenge. However, recent data show that removal of Tregs can tip the balance in favor of the generation of radiation-induced anti-tumor immunity. The clinical challenge is to do so without excessive depletion that might precipitate serious autoimmune reactions and increase the likelihood of normal tissue complications. The selective modulation of Treg biology to maintain immune tolerance and control of normal tissue damage, while releasing the "brakes" on anti-tumor immune responses, is a worthy aim with promise for enhancing the therapeutic benefit of RT for cancer.
Keywords: Tregs; danger; radiation.
Figures
Similar articles
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
-
STAT3 Modulation of Regulatory T Cells in Response to Radiation Therapy in Head and Neck Cancer.J Natl Cancer Inst. 2019 Dec 1;111(12):1339-1349. doi: 10.1093/jnci/djz036. J Natl Cancer Inst. 2019. PMID: 30863843 Free PMC article.
-
Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy.Immunol Rev. 2008 Aug;224:141-65. doi: 10.1111/j.1600-065X.2008.00649.x. Immunol Rev. 2008. PMID: 18759925 Review.
-
The role of regulatory T Cells in autoimmune orchitis.Andrologia. 2018 Dec;50(11):e13092. doi: 10.1111/and.13092. Andrologia. 2018. PMID: 30569653 Review.
-
Self, non-self, and danger: a complementary view.Adv Exp Med Biol. 2006;586:71-94. doi: 10.1007/0-387-34134-X_6. Adv Exp Med Biol. 2006. PMID: 16893066 Review.
Cited by
-
Immunomodulatory effects of carbon ion radiotherapy in patients with localized prostate cancer.J Cancer Res Clin Oncol. 2023 Jul;149(8):4533-4545. doi: 10.1007/s00432-022-04194-9. Epub 2022 Sep 23. J Cancer Res Clin Oncol. 2023. PMID: 36138265 Free PMC article.
-
Strategies for optimizing the response of cancer and normal tissues to radiation.Nat Rev Drug Discov. 2013 Jul;12(7):526-42. doi: 10.1038/nrd4003. Nat Rev Drug Discov. 2013. PMID: 23812271 Free PMC article. Review.
-
Normal tissue damage: its importance, history and challenges for the future.Br J Radiol. 2019 Jan;92(1093):20180048. doi: 10.1259/bjr.20180048. Epub 2018 Apr 9. Br J Radiol. 2019. PMID: 29616836 Free PMC article. Review.
-
Harnessing the Immunological Effects of Radiation to Improve Immunotherapies in Cancer.Int J Mol Sci. 2023 Apr 16;24(8):7359. doi: 10.3390/ijms24087359. Int J Mol Sci. 2023. PMID: 37108522 Free PMC article. Review.
-
Combining radiotherapy and cancer immunotherapy: a paradigm shift.J Natl Cancer Inst. 2013 Feb 20;105(4):256-65. doi: 10.1093/jnci/djs629. Epub 2013 Jan 4. J Natl Cancer Inst. 2013. PMID: 23291374 Free PMC article. Review.
References
-
- Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M. C., Ullrich E., Saulnier P., Yang H., Amigorena S., Ryffel B., Barrat F. J., Saftig P., Levi F., Lidereau R., Nogues C., Mira J. P., Chompret A., Joulin V., Clavel-Chapelon F., Bourhis J., Andre F., Delaloge S., Tursz T., Kroemer G., Zitvogel L. (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–105910.1038/nm1622 - DOI - PubMed
-
- Awwad M., North R. J. (1989). Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 49, 1649–1654 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

