Mio/dChREBP coordinately increases fat mass by regulating lipid synthesis and feeding behavior in Drosophila
- PMID: 22910416
- PMCID: PMC3445662
- DOI: 10.1016/j.bbrc.2012.08.028
Mio/dChREBP coordinately increases fat mass by regulating lipid synthesis and feeding behavior in Drosophila
Abstract
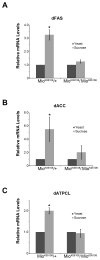
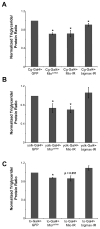
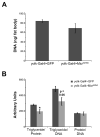
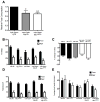
During nutrient excess, triglycerides are synthesized and stored to provide energy during times of famine. The presence of high glucose leads to the activation of carbohydrate response element binding protein (ChREBP), a transcription factor that induces the expression of a number of glycolytic and lipogenic enzymes. ChREBP is expressed in major metabolic tissues and while we have a basic understanding of ChREBP function in liver, in vivo genetic systems to study the function of ChREBP in other tissues are lacking. In this study, we characterized the role of the Drosophila homolog of ChREBP, Mlx interactor (Mio), in controlling fat accumulation in larvae and adult flies. In Mio mutants, high sugar-induced lipogenic enzyme mRNA expression is blunted and lowering Mio levels specifically in the fat body using RNA interference leads to a lean phenotype. A lean phenotype is also observed when the gene bigmax, the fly homolog of ChREBP's binding partner Mlx, is decreased in the larval fat body. Interestingly, depleting Mio in the fat body results in decreased feeding providing a potential cause of the lowered triglycerides observed in these animals. However, Mio does not seem to function as a general regulator of hunger-induced behaviors as decreasing fat body Mio levels has no effect on sleep under fed or starved conditions. Together, these data implicate a role for Mio in controlling fat accumulation in Drosophila and suggests that it may act as a nutrient sensor in the fat body to coordinate feeding behavior with nutrient availability.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Mio acts in the Drosophila brain to control nutrient storage and feeding.Gene. 2015 Sep 1;568(2):190-5. doi: 10.1016/j.gene.2015.05.055. Epub 2015 May 27. Gene. 2015. PMID: 26024590 Free PMC article.
-
The Regulation of Muscle Structure and Metabolism by Mio/dChREBP in Drosophila.PLoS One. 2015 Aug 25;10(8):e0136504. doi: 10.1371/journal.pone.0136504. eCollection 2015. PLoS One. 2015. PMID: 26305467 Free PMC article.
-
Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila.PLoS Genet. 2013 Apr;9(4):e1003438. doi: 10.1371/journal.pgen.1003438. Epub 2013 Apr 4. PLoS Genet. 2013. PMID: 23593032 Free PMC article.
-
Sweet Sixteenth for ChREBP: Established Roles and Future Goals.Cell Metab. 2017 Aug 1;26(2):324-341. doi: 10.1016/j.cmet.2017.07.004. Cell Metab. 2017. PMID: 28768172 Review.
-
ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome.Endocr J. 2008 Aug;55(4):617-24. doi: 10.1507/endocrj.k07e-110. Epub 2008 May 19. Endocr J. 2008. PMID: 18490833 Review.
Cited by
-
Ade2 Functions in the Drosophila Fat Body To Promote Sleep.G3 (Bethesda). 2018 Nov 6;8(11):3385-3395. doi: 10.1534/g3.118.200554. G3 (Bethesda). 2018. PMID: 30249751 Free PMC article.
-
Regulation of Carbohydrate Energy Metabolism in Drosophila melanogaster.Genetics. 2017 Dec;207(4):1231-1253. doi: 10.1534/genetics.117.199885. Genetics. 2017. PMID: 29203701 Free PMC article. Review.
-
Metabolic gene regulation by Drosophila GATA transcription factor Grain.PLoS Genet. 2021 Oct 11;17(10):e1009855. doi: 10.1371/journal.pgen.1009855. eCollection 2021 Oct. PLoS Genet. 2021. PMID: 34634038 Free PMC article.
-
Altering the sex determination pathway in Drosophila fat body modifies sex-specific stress responses.Am J Physiol Regul Integr Comp Physiol. 2014 Jul 1;307(1):R82-92. doi: 10.1152/ajpregu.00003.2014. Epub 2014 Apr 30. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24789992 Free PMC article.
-
The fat body cortical actin network regulates Drosophila inter-organ nutrient trafficking, signaling, and adipose cell size.Elife. 2023 May 5;12:e81170. doi: 10.7554/eLife.81170. Elife. 2023. PMID: 37144872 Free PMC article.
References
-
- Towle HC, Kaytor EN, Shih HM. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annual review of nutrition. 1997;17:405–433. - PubMed
-
- Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, Ratziu V, Serfaty L, Housset C, Capeau J, Girard J, Guillou H, Postic C. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. The Journal of clinical investigation. 2012;122:2176–2194. - PMC - PubMed
-
- Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annual review of nutrition. 2007;27:179–192. - PubMed
-
- Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. The Journal of biological chemistry. 2004;279:15662–15669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

