A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface
- PMID: 22909819
- PMCID: PMC3474920
- DOI: 10.1038/emboj.2012.235
A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface
Abstract
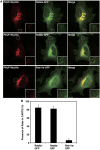
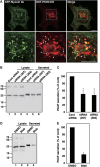
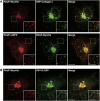
We have isolated a membrane fraction enriched in a class of transport carriers that form at the trans Golgi network (TGN) and are destined for the cell surface in HeLa cells. Protein kinase D (PKD) is required for the biogenesis of these carriers that contain myosin II, Rab6a, Rab8a, and synaptotagmin II, as well as a number of secretory and plasma membrane-specific cargoes. Our findings reveal a requirement for myosin II in the migration of these transport carriers but not in their biogenesis per se. Based on the cargo secreted by these carriers we have named them CARTS for CARriers of the TGN to the cell Surface. Surprisingly, CARTS are distinct from the carriers that transport vesicular stomatitis virus (VSV)-G protein and collagen I from the TGN to the cell surface. Altogether, the identification of CARTS provides a valuable means to understand TGN to cell surface traffic.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
Cargo carriers from the Golgi to the cell surface.EMBO J. 2012 Oct 17;31(20):3954-5. doi: 10.1038/emboj.2012.249. Epub 2012 Aug 31. EMBO J. 2012. PMID: 22940689 Free PMC article.
Similar articles
-
Cargo carriers from the Golgi to the cell surface.EMBO J. 2012 Oct 17;31(20):3954-5. doi: 10.1038/emboj.2012.249. Epub 2012 Aug 31. EMBO J. 2012. PMID: 22940689 Free PMC article.
-
Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network.Cell. 2001 Feb 9;104(3):409-20. doi: 10.1016/s0092-8674(01)00228-8. Cell. 2001. PMID: 11239398
-
The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers.Cells. 2021 Jun 28;10(7):1618. doi: 10.3390/cells10071618. Cells. 2021. PMID: 34203456 Free PMC article. Review.
-
Dimeric PKD regulates membrane fission to form transport carriers at the TGN.J Cell Biol. 2007 Dec 17;179(6):1123-31. doi: 10.1083/jcb.200703166. J Cell Biol. 2007. PMID: 18086912 Free PMC article.
-
The formation of TGN-to-plasma-membrane transport carriers.Annu Rev Cell Dev Biol. 2006;22:439-55. doi: 10.1146/annurev.cellbio.21.012704.133126. Annu Rev Cell Dev Biol. 2006. PMID: 16824007 Review.
Cited by
-
The tumor suppressor protein DLC1 maintains protein kinase D activity and Golgi secretory function.J Biol Chem. 2018 Sep 14;293(37):14407-14416. doi: 10.1074/jbc.RA118.003787. Epub 2018 Jul 25. J Biol Chem. 2018. PMID: 30045871 Free PMC article.
-
Retrograde transport of CDMPR depends on several machineries as analyzed by sulfatable nanobodies.Life Sci Alliance. 2022 Mar 21;5(7):e202101269. doi: 10.26508/lsa.202101269. Print 2022 Mar. Life Sci Alliance. 2022. PMID: 35314489 Free PMC article.
-
Type I IFN drives unconventional IL-1β secretion in lupus monocytes.Immunity. 2024 Nov 12;57(11):2497-2513.e12. doi: 10.1016/j.immuni.2024.09.004. Epub 2024 Oct 7. Immunity. 2024. PMID: 39378884
-
A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network.Elife. 2013 Jan 8;2:e00160. doi: 10.7554/eLife.00160. Elife. 2013. PMID: 23326640 Free PMC article.
-
Sphingomyelin homeostasis is required to form functional enzymatic domains at the trans-Golgi network.J Cell Biol. 2014 Sep 1;206(5):609-18. doi: 10.1083/jcb.201405009. J Cell Biol. 2014. PMID: 25179630 Free PMC article.
References
-
- Banting G, Ponnambalam S (1997) TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim Biophys Acta 1355: 209–217 - PubMed
-
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V (2006) Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439: 604–607 - PubMed
-
- Bard F, Malhotra V (2006) The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol 22: 439–455 - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

