Rapid development of a potent photo-triggered inhibitor of the serine hydrolase RBBP9
- PMID: 22907802
- PMCID: PMC3777423
- DOI: 10.1002/cbic.201200445
Rapid development of a potent photo-triggered inhibitor of the serine hydrolase RBBP9
Abstract
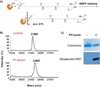
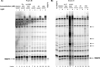
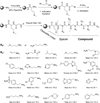
The serine hydrolases constitute a large class of enzymes that play important roles in physiology. There is great interest in the development of potent and selective pharmacological inhibitors of these proteins. Traditional active-site inhibitors often have limited selectivity within this superfamily and are tedious and expensive to discover. Using the serine hydrolase RBBP9 as a model target, we designed a rapid and relatively inexpensive route to highly selective peptoid-based inhibitors that can be activated by visible light. This technology provides rapid access to photo-activated tool compounds capable of selectively blocking the function of particular serine hydrolases.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






Similar articles
-
Oxime esters as selective, covalent inhibitors of the serine hydrolase retinoblastoma-binding protein 9 (RBBP9).Bioorg Med Chem Lett. 2010 Apr 1;20(7):2254-8. doi: 10.1016/j.bmcl.2010.02.011. Epub 2010 Feb 6. Bioorg Med Chem Lett. 2010. PMID: 20207142 Free PMC article.
-
Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation.Int J Mol Sci. 2020 Nov 26;21(23):8983. doi: 10.3390/ijms21238983. Int J Mol Sci. 2020. PMID: 33256189 Free PMC article.
-
Chemoproteomic Identification of Serine Hydrolase RBBP9 as a Valacyclovir-Activating Enzyme.Mol Pharm. 2020 May 4;17(5):1706-1714. doi: 10.1021/acs.molpharmaceut.0c00131. Epub 2020 Mar 30. Mol Pharm. 2020. PMID: 32196348
-
Human retinoblastoma binding protein 9, a serine hydrolase implicated in pancreatic cancers.Protein Pept Lett. 2012 Feb;19(2):194-7. doi: 10.2174/092986612799080356. Protein Pept Lett. 2012. PMID: 21933118 Free PMC article. Review.
-
Identifying inhibitors of ATM and ATR kinase activities.Methods Mol Med. 2003;85:49-56. doi: 10.1385/1-59259-380-1:49. Methods Mol Med. 2003. PMID: 12710196 Review. No abstract available.
Cited by
-
Combination of Ru(ii) complexes and light: new frontiers in cancer therapy.Chem Sci. 2015 May 1;6(5):2660-2686. doi: 10.1039/c4sc03759f. Epub 2015 Jan 13. Chem Sci. 2015. PMID: 29308166 Free PMC article.
-
Towards vast libraries of scaffold-diverse, conformationally constrained oligomers.Chem Commun (Camb). 2016 May 4;52(36):6038-59. doi: 10.1039/c6cc00617e. Epub 2016 Mar 21. Chem Commun (Camb). 2016. PMID: 26996593 Free PMC article.
-
Solid phase synthesis of 1,3,4-oxadiazin-5 (6R)-one and 1,3,4-oxadiazol-2-one scaffolds from acyl hydrazides.Org Biomol Chem. 2015 Jan 7;13(1):59-63. doi: 10.1039/c4ob01883d. Org Biomol Chem. 2015. PMID: 25354697 Free PMC article.
-
Optical manipulation of molecular function by chromophore-assisted light inactivation.Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(4):197-209. doi: 10.2183/pjab.97.011. Proc Jpn Acad Ser B Phys Biol Sci. 2021. PMID: 33840676 Free PMC article. Review.
-
Photodynamic Therapy by Glucose Transporter 1-Selective Light Inactivation.ACS Omega. 2022 Sep 16;7(38):34685-34692. doi: 10.1021/acsomega.2c05042. eCollection 2022 Sep 27. ACS Omega. 2022. PMID: 36188330 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

