The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation
- PMID: 22904198
- PMCID: PMC3547100
- DOI: 10.1165/rcmb.2011-0418OC
The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation
Abstract
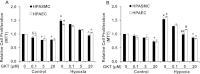
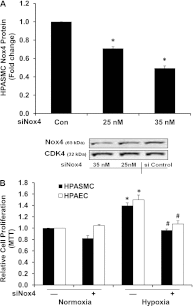
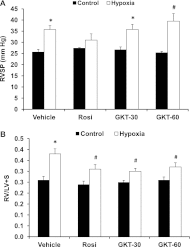
Increased NADP reduced (NADPH) oxidase 4 (Nox4) and reduced expression of the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) contribute to hypoxia-induced pulmonary hypertension (PH). To examine the role of Nox4 activity in pulmonary vascular cell proliferation and PH, the current study used a novel Nox4 inhibitor, GKT137831, in hypoxia-exposed human pulmonary artery endothelial or smooth muscle cells (HPAECs or HPASMCs) in vitro and in hypoxia-treated mice in vivo. HPAECs or HPASMCs were exposed to normoxia or hypoxia (1% O(2)) for 72 hours with or without GKT137831. Cell proliferation and Nox4, PPARγ, and transforming growth factor (TGF)β1 expression were measured. C57Bl/6 mice were exposed to normoxia or hypoxia (10% O(2)) for 3 weeks with or without GKT137831 treatment during the final 10 days of exposure. Lung PPARγ and TGF-β1 expression, right ventricular hypertrophy (RVH), right ventricular systolic pressure (RVSP), and pulmonary vascular remodeling were measured. GKT137831 attenuated hypoxia-induced H(2)O(2) release, proliferation, and TGF-β1 expression and blunted reductions in PPARγ in HPAECs and HPASMCs in vitro. In vivo GKT137831 inhibited hypoxia-induced increases in TGF-β1 and reductions in PPARγ expression and attenuated RVH and pulmonary artery wall thickness but not increases in RVSP or muscularization of small arterioles. This study shows that Nox4 plays a critical role in modulating proliferative responses of pulmonary vascular wall cells. Targeting Nox4 with GKT137831 provides a novel strategy to attenuate hypoxia-induced alterations in pulmonary vascular wall cells that contribute to vascular remodeling and RVH, key features involved in PH pathogenesis.
Figures
Similar articles
-
Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells.Free Radic Biol Med. 2013 Oct;63:151-60. doi: 10.1016/j.freeradbiomed.2013.05.013. Epub 2013 May 15. Free Radic Biol Med. 2013. PMID: 23684777 Free PMC article.
-
PPAR{gamma} regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-{kappa}B.Am J Physiol Lung Cell Mol Physiol. 2010 Oct;299(4):L559-66. doi: 10.1152/ajplung.00090.2010. Epub 2010 Jul 9. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20622120 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma depletion stimulates Nox4 expression and human pulmonary artery smooth muscle cell proliferation.Free Radic Biol Med. 2015 Mar;80:111-20. doi: 10.1016/j.freeradbiomed.2014.12.019. Epub 2014 Dec 31. Free Radic Biol Med. 2015. PMID: 25557278 Free PMC article.
-
PPARγ and RhoBTB1 in hypertension.Curr Opin Nephrol Hypertens. 2020 Mar;29(2):161-170. doi: 10.1097/MNH.0000000000000579. Curr Opin Nephrol Hypertens. 2020. PMID: 31789920 Free PMC article. Review.
-
The Role of PPARgamma in pulmonary vascular disease.J Investig Med. 2008 Feb;56(2):518-21. doi: 10.2310/JIM.0b013e318165e921. J Investig Med. 2008. PMID: 18317434 Free PMC article. Review.
Cited by
-
NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways.Circulation. 2015 Feb 17;131(7):643-55. doi: 10.1161/CIRCULATIONAHA.114.011079. Epub 2015 Jan 14. Circulation. 2015. PMID: 25589557 Free PMC article.
-
Intermittent short-duration reoxygenation relieves high-altitude pulmonary hypertension via NOX4/H2O2/PPAR-γ axis.Clin Sci (Lond). 2024 Feb 7;138(3):103-115. doi: 10.1042/CS20231508. Clin Sci (Lond). 2024. PMID: 38237016 Free PMC article.
-
Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis.Front Pharmacol. 2022 Jan 21;12:794997. doi: 10.3389/fphar.2021.794997. eCollection 2021. Front Pharmacol. 2022. PMID: 35126133 Free PMC article. Review.
-
Beneficial effects of fenofibrate in pulmonary hypertension in rats.Mol Cell Biochem. 2018 Dec;449(1-2):185-194. doi: 10.1007/s11010-018-3355-3. Epub 2018 May 14. Mol Cell Biochem. 2018. PMID: 29761247
-
Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes.Redox Biol. 2018 Jun;16:332-343. doi: 10.1016/j.redox.2018.03.011. Epub 2018 Mar 17. Redox Biol. 2018. PMID: 29587244 Free PMC article.
References
-
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163 - PubMed
-
- Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002;105:1429–1435 - PubMed
-
- Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 2006;8:691–728 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

