Iron chelators with topoisomerase-inhibitory activity and their anticancer applications
- PMID: 22900902
- PMCID: PMC3557438
- DOI: 10.1089/ars.2012.4877
Iron chelators with topoisomerase-inhibitory activity and their anticancer applications
Abstract
Significance: Iron and topoisomerases are abundant and essential cellular components. Iron is required for several key processes such as DNA synthesis, mitochondrial electron transport, synthesis of heme, and as a co-factor for many redox enzymes. Topoisomerases serve as critical enzymes that resolve topological problems during DNA synthesis, transcription, and repair. Neoplastic cells have higher uptake and utilization of iron, as well as elevated levels of topoisomerase family members. Separately, the chelation of iron and the cytotoxic inhibition of topoisomerase have yielded potent anticancer agents.
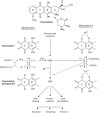
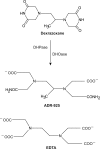
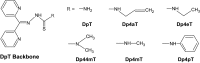
Recent advances: The chemotherapeutic drugs doxorubicin and dexrazoxane both chelate iron and target topoisomerase 2 alpha (top2α). Newer chelators such as di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone and thiosemicarbazone -24 have recently been identified as top2α inhibitors. The growing list of agents that appear to chelate iron and inhibit topoisomerases prompts the question of whether and how these two distinct mechanisms might interplay for a cytotoxic chemotherapeutic outcome.
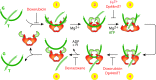
Critical issues: While iron chelation and topoisomerase inhibition each represent mechanistically advantageous anticancer therapeutic strategies, dual targeting agents present an attractive multi-modal opportunity for enhanced anticancer tumor killing and overcoming drug resistance. The commonalities and caveats of dual inhibition are presented in this review.
Future directions: Gaps in knowledge, relevant biomarkers, and strategies for future in vivo studies with dual inhibitors are discussed.
Figures
Similar articles
-
Discovery and Development of Topoisomerase Inhibitors as Anticancer Agents.Mini Rev Med Chem. 2016;16(15):1219-1229. doi: 10.2174/1389557516666160822110819. Mini Rev Med Chem. 2016. PMID: 27549098 Review.
-
The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells.Cancer Res. 2009 Feb 1;69(3):948-57. doi: 10.1158/0008-5472.CAN-08-1437. Epub 2009 Jan 27. Cancer Res. 2009. PMID: 19176392 Free PMC article.
-
DNA binding and Topoisomerase inhibition: How can these mechanisms be explored to design more specific anticancer agents?Biomed Pharmacother. 2017 Dec;96:1538-1556. doi: 10.1016/j.biopha.2017.11.054. Epub 2017 Nov 22. Biomed Pharmacother. 2017. PMID: 29174576 Review.
-
Topoisomerases and Anthracyclines: Recent Advances and Perspectives in Anticancer Therapy and Prevention of Cardiotoxicity.Curr Med Chem. 2017;24(15):1607-1626. doi: 10.2174/0929867323666161214120355. Curr Med Chem. 2017. PMID: 27978799 Review.
-
The medicinal chemistry of novel iron chelators for the treatment of cancer.Curr Top Med Chem. 2011;11(5):483-99. doi: 10.2174/156802611794785190. Curr Top Med Chem. 2011. PMID: 21192781 Review.
Cited by
-
Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway.Acta Pharm Sin B. 2019 Jul;9(4):690-701. doi: 10.1016/j.apsb.2019.03.003. Epub 2019 Mar 16. Acta Pharm Sin B. 2019. PMID: 31384530 Free PMC article.
-
Interplay Between Iron Overload and Osteoarthritis: Clinical Significance and Cellular Mechanisms.Front Cell Dev Biol. 2022 Jan 14;9:817104. doi: 10.3389/fcell.2021.817104. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35096841 Free PMC article. Review.
-
Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity.Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):2011-6. doi: 10.1073/pnas.1321783111. Epub 2014 Jan 21. Proc Natl Acad Sci U S A. 2014. PMID: 24449919 Free PMC article.
-
G2/M Cell Cycle Arrest and Tumor Selective Apoptosis of Acute Leukemia Cells by a Promising Benzophenone Thiosemicarbazone Compound.PLoS One. 2015 Sep 11;10(9):e0136878. doi: 10.1371/journal.pone.0136878. eCollection 2015. PLoS One. 2015. PMID: 26360247 Free PMC article.
-
Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation.J Clin Invest. 2014 Feb;124(2):617-30. doi: 10.1172/JCI72931. Epub 2014 Jan 2. J Clin Invest. 2014. PMID: 24382354 Free PMC article.
References
-
- Abou El Hassan MA. Heijn M. Rabelink MJ. van der Vijgh WJ. Bast A. Hoeben RC. The protective effect of cardiac gene transfer of CuZn-sod in comparison with the cardioprotector monohydroxyethylrutoside against doxorubicin-induced cardiotoxicity in cultured cells. Cancer Gene Ther. 2003;10:270–277. - PubMed
-
- Aisen P. Wessling-Resnick M. Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200–206. - PubMed
-
- Akimitsu N. Adachi N. Hirai H. Hossain MS. Hamamoto H. Kobayashi M. Aratani Y. Koyama H. Sekimizu K. Enforced cytokinesis without complete nuclear division in embryonic cells depleting the activity of DNA topoisomerase IIalpha. Genes Cells. 2003;8:393–402. - PubMed
-
- Aluise CD. Miriyala S. Noel T. Sultana R. Jungsuwadee P. Taylor TJ. Cai J. Pierce WM. Vore M. Moscow JA. St. Clair DK. Butterfield DA. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50:1630–1638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

