Current management of parainfluenza pneumonitis in immunocompromised patients: a review
- PMID: 22893749
- PMCID: PMC3418768
- DOI: 10.2147/IDR.S25874
Current management of parainfluenza pneumonitis in immunocompromised patients: a review
Abstract
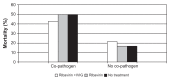
Parainfluenza viruses (PIV) are common respiratory viruses that belong to the Paramyxoviridae family. PIV infection can lead to a wide variety of clinical syndromes ranging from mild upper respiratory illness to severe pneumonia. Severe disease can be seen in elderly or chronically ill persons and may be fatal in persons with compromised immune systems, particularly children with severe combined immunodeficiency disease syndrome and hematopathic stem cell transplant recipients. At present, there are no licensed antiviral agents for the treatment of PIV infection. Aerosolized or systemic ribavirin in combination with intravenous gamma globulin has been reported in small, uncontrolled series and case reports of immunocompromised patients. A number of agents show antiviral activity in vitro and in animals, but none are currently approved for human use.
Keywords: antiviral agents; immunocompromised host; parainfluenza virus.
Figures
Similar articles
-
Respiratory syncytial virus and parainfluenza virus infections in the immunocompromised host.Semin Respir Infect. 1995 Dec;10(4):224-31. Semin Respir Infect. 1995. PMID: 8668850 Review.
-
Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children.Pediatrics. 2002 Jul;110(1 Pt 1):e9. doi: 10.1542/peds.110.1.e9. Pediatrics. 2002. PMID: 12093990 Review.
-
Parainfluenza Virus in the Hospitalized Adult.Clin Infect Dis. 2017 Oct 16;65(9):1570-1576. doi: 10.1093/cid/cix528. Clin Infect Dis. 2017. PMID: 28591775 Review.
-
Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome.Blood. 2001 Aug 1;98(3):573-8. doi: 10.1182/blood.v98.3.573. Blood. 2001. PMID: 11468152
-
Prevention and treatment of respiratory syncytial virus and parainfluenza viruses in immunocompromised patients.Am J Med. 1997 Mar 17;102(3A):61-70; discussion 75-6. doi: 10.1016/s0002-9343(97)00014-4. Am J Med. 1997. PMID: 10868145 Review.
Cited by
-
Epidemiology of Human Parainfluenza Virus Infections among Pediatric Patients in Hainan Island, China, 2021-2023.Pathogens. 2024 Aug 30;13(9):740. doi: 10.3390/pathogens13090740. Pathogens. 2024. PMID: 39338931 Free PMC article.
-
Early initiation of venovenous extracorporeal membrane oxygenation in a mechanically ventilated patient with severe acute respiratory distress syndrome.BMJ Case Rep. 2018 Oct 12;2018:bcr2018226223. doi: 10.1136/bcr-2018-226223. BMJ Case Rep. 2018. PMID: 30317205 Free PMC article.
-
Inhibition of primary clinical isolates of human parainfluenza virus by DAS181 in cell culture and in a cotton rat model.Antiviral Res. 2013 Nov;100(2):562-6. doi: 10.1016/j.antiviral.2013.09.014. Epub 2013 Sep 27. Antiviral Res. 2013. PMID: 24076357 Free PMC article.
-
Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome.Clin Infect Dis. 2014 May;58(10):1357-68. doi: 10.1093/cid/ciu134. Epub 2014 Mar 5. Clin Infect Dis. 2014. PMID: 24599766 Free PMC article.
-
Prophylactic Measures During Induction for Acute Myeloid Leukemia.Curr Oncol Rep. 2017 Mar;19(3):18. doi: 10.1007/s11912-017-0574-9. Curr Oncol Rep. 2017. PMID: 28251490 Review.
References
-
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928. - PubMed
-
- Stankova J, Carret AS, Moore D, et al. Long-term therapy with aerosolized ribavirin for parainfluenza 3 virus respiratory tract infection in an infant with severe combined immunodeficiency. Pediatr Transplant. 2007;11(2):209–213. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

