Ribavirin modulates the conversion of human CD4(+) CD25(-) T cell to CD4(+) CD25(+) FOXP3(+) T cell via suppressing interleukin-10-producing regulatory T cell
- PMID: 22891772
- PMCID: PMC3482683
- DOI: 10.1111/imm.12005
Ribavirin modulates the conversion of human CD4(+) CD25(-) T cell to CD4(+) CD25(+) FOXP3(+) T cell via suppressing interleukin-10-producing regulatory T cell
Abstract
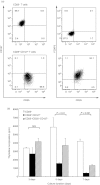
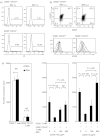
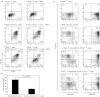
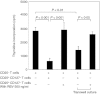
Because regulatory T (Treg) cells play an important role in modulating the immune system response against both endogenous and exogenous antigens, their control is critical to establish immunotherapy against autoimmune disorders, chronic viral infections and tumours. Ribavirin (RBV), an antiviral reagent used with interferon, is known to polarize the T helper (Th) 1/2 cell balance toward Th1 cells. Although the immunoregulatory mechanisms of RBV are not fully understood, it has been expected that RBV would affect T reg cells to modulate the Th1/2 cell balance. To confirm this hypothesis, we investigated whether RBV modulates the inhibitory activity of human peripheral CD4(+) CD25(+) CD127(-) T cells in vitro. CD4(+) CD25(+) CD127(-) T cells pre-incubated with RBV lose their ability to inhibit the proliferation of CD4(+) CD25(-) T cells. Expression of Forkhead box P3 (FOXP3) in CD4(+) CD25(-) T cells was down-modulated when they were incubated with CD4(+) CD25(+) CD127(-) T cells pre-incubated with RBV without down-modulating CD45RO on their surface. In addition, transwell assays and cytokine-neutralizing assays revealed that this effect depended mainly on the inhibition of interleukin-10 (IL-10) produced from CD4(+) CD25(+) CD127(-) T cells. These results indicated that RBV might inhibit the conversion of CD4(+) CD25(-) FOXP3(-) naive T cells into CD4(+) CD25(+) FOXP3(+) adaptive Treg cells by down-modulating the IL-10-producing Treg 1 cells to prevent these effector T cells from entering anergy and to maintain Th1 cell activity. Taken together, our findings suggest that RBV would be useful for both elimination of long-term viral infections such as hepatitis C virus infection and for up-regulation of tumour-specific cellular immune responses to prevent carcinogenesis, especially hepatocellular carcinoma.
© 2012 The Authors. Immunology © 2012 Blackwell Publishing Ltd.
Figures

Similar articles
-
Comparative analyses of regulatory T cell subsets in patients with hepatocellular carcinoma: a crucial role of CD25(-) FOXP3(-) T cells.Int J Cancer. 2012 Dec 1;131(11):2573-83. doi: 10.1002/ijc.27535. Epub 2012 Mar 29. Int J Cancer. 2012. PMID: 22419479
-
Interleukin-7 matures suppressive CD127(+) forkhead box P3 (FoxP3)(+) T cells into CD127(-) CD25(high) FoxP3(+) regulatory T cells.Clin Exp Immunol. 2011 Jul;165(1):60-76. doi: 10.1111/j.1365-2249.2011.04334.x. Epub 2011 Mar 17. Clin Exp Immunol. 2011. PMID: 21413939 Free PMC article.
-
CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression.Clin Immunol. 2009 Apr;131(1):109-18. doi: 10.1016/j.clim.2008.11.010. Epub 2009 Jan 18. Clin Immunol. 2009. PMID: 19153062
-
Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases.Nat Rev Immunol. 2010 Dec;10(12):849-59. doi: 10.1038/nri2889. Nat Rev Immunol. 2010. PMID: 21107346 Free PMC article. Review.
-
CD4+CD25+Foxp3+ regulatory T cells: from basic research to potential therapeutic use.Swiss Med Wkly. 2007 Nov 17;137(45-46):625-34. doi: 10.4414/smw.2007.11916. Swiss Med Wkly. 2007. PMID: 18027108 Review.
Cited by
-
Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: a prospective multicentre study.J Transl Med. 2019 Aug 28;17(1):292. doi: 10.1186/s12967-019-2033-x. J Transl Med. 2019. PMID: 31462268 Free PMC article.
-
Lipopolysaccharide from Rhodobacter sphaeroides Attenuates Microglia-Mediated Inflammation and Phagocytosis and Directs Regulatory T Cell Response.Int J Inflam. 2015;2015:361326. doi: 10.1155/2015/361326. Epub 2015 Sep 17. Int J Inflam. 2015. PMID: 26457222 Free PMC article.
-
Vaccines and Therapeutics Against Hantaviruses.Front Microbiol. 2020 Jan 30;10:2989. doi: 10.3389/fmicb.2019.02989. eCollection 2019. Front Microbiol. 2020. PMID: 32082263 Free PMC article. Review.
-
The Impact of Steatosis on Chronic Hepatitis C Progression and Response to Antiviral Treatments.Biomedicines. 2021 Oct 17;9(10):1491. doi: 10.3390/biomedicines9101491. Biomedicines. 2021. PMID: 34680608 Free PMC article. Review.
-
Expression and significance of CD4(+)CD25(+)CD127(-) regulatory T cells in peripheral blood of patients with different phenotypes of Guillain-Barré syndrome.Int J Clin Exp Med. 2015 Oct 15;8(10):19126-31. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26770543 Free PMC article.
References
-
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–8. - PubMed
-
- Rosen HR, Miner C, Sasaki AW, Lewinsohn DM, Conrad AJ, Bakke A, Bouwer HG, Hinrichs DJ. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology. 2002;35:190–8. - PubMed
-
- Osna N, Silonova G, Vilgert N, et al. Chronic hepatitis C: T-helper1/T-helper2 imbalance could cause virus persistence in peripheral blood. Scand J Clin Lab Invest. 1997;57:703–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

