Is the amyloid hypothesis of Alzheimer's disease therapeutically relevant?
- PMID: 22891628
- PMCID: PMC3686157
- DOI: 10.1042/BJ20120653
Is the amyloid hypothesis of Alzheimer's disease therapeutically relevant?
Abstract
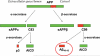
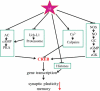
The conventional view of AD (Alzheimer's disease) is that much of the pathology is driven by an increased load of β-amyloid in the brain of AD patients (the 'Amyloid Hypothesis'). Yet, many therapeutic strategies based on lowering β-amyloid have so far failed in clinical trials. This failure of β-amyloid-lowering agents has caused many to question the Amyloid Hypothesis itself. However, AD is likely to be a complex disease driven by multiple factors. In addition, it is increasingly clear that β-amyloid processing involves many enzymes and signalling pathways that play a role in a diverse array of cellular processes. Thus the clinical failure of β-amyloid-lowering agents does not mean that the hypothesis itself is incorrect; it may simply mean that manipulating β-amyloid directly is an unrealistic strategy for therapeutic intervention, given the complex role of β-amyloid in neuronal physiology. Another possible problem may be that toxic β-amyloid levels have already caused irreversible damage to downstream cellular pathways by the time dementia sets in. We argue in the present review that a more direct (and possibly simpler) approach to AD therapeutics is to rescue synaptic dysfunction directly, by focusing on the mechanisms by which elevated levels of β-amyloid disrupt synaptic physiology.
Figures
Similar articles
-
Targeting histone-modifications in Alzheimer's disease. What is the evidence that this is a promising therapeutic avenue?Neuropharmacology. 2014 May;80:95-102. doi: 10.1016/j.neuropharm.2014.01.038. Epub 2014 Jan 31. Neuropharmacology. 2014. PMID: 24486385 Review.
-
Beyond amyloid: getting real about nonamyloid targets in Alzheimer's disease.Alzheimers Dement. 2013 Jul;9(4):452-458.e1. doi: 10.1016/j.jalz.2013.01.017. Alzheimers Dement. 2013. PMID: 23809366 Free PMC article.
-
Amyloid-β Receptors: The Good, the Bad, and the Prion Protein.J Biol Chem. 2016 Feb 12;291(7):3174-83. doi: 10.1074/jbc.R115.702704. Epub 2015 Dec 30. J Biol Chem. 2016. PMID: 26719327 Free PMC article. Review.
-
Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?J Biol Chem. 2018 Oct 5;293(40):15419-15428. doi: 10.1074/jbc.R118.003999. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143530 Free PMC article. Review.
-
Do anti-amyloid-β drugs affect neuropsychiatric status in Alzheimer's disease patients?Ageing Res Rev. 2019 Nov;55:100948. doi: 10.1016/j.arr.2019.100948. Epub 2019 Aug 24. Ageing Res Rev. 2019. PMID: 31454563 Review.
Cited by
-
Re-engineering a neuroprotective, clinical drug as a procognitive agent with high in vivo potency and with GABAA potentiating activity for use in dementia.BMC Neurosci. 2015 Oct 19;16:67. doi: 10.1186/s12868-015-0208-9. BMC Neurosci. 2015. PMID: 26480871 Free PMC article.
-
The role and therapeutic targeting of α-, β- and γ-secretase in Alzheimer's disease.Future Sci OA. 2015 Nov 1;1(3):FSO11. doi: 10.4155/fso.15.9. eCollection 2015 Nov. Future Sci OA. 2015. PMID: 28031886 Free PMC article. Review.
-
Development of Novel In Vivo Chemical Probes to Address CNS Protein Kinase Involvement in Synaptic Dysfunction.PLoS One. 2013 Jun 26;8(6):e66226. doi: 10.1371/journal.pone.0066226. Print 2013. PLoS One. 2013. PMID: 23840427 Free PMC article.
-
Development of a Robust High-Throughput Screening Platform for Inhibitors of the Striatal-Enriched Tyrosine Phosphatase (STEP).Int J Mol Sci. 2021 Apr 23;22(9):4417. doi: 10.3390/ijms22094417. Int J Mol Sci. 2021. PMID: 33922601 Free PMC article.
-
An insight into the concept of neuroinflammation and neurodegeneration in Alzheimer's disease: targeting molecular approach Nrf2, NF-κB, and CREB.Inflammopharmacology. 2024 Oct;32(5):2943-2960. doi: 10.1007/s10787-024-01502-2. Epub 2024 Jun 29. Inflammopharmacology. 2024. PMID: 38951436 Review.
References
-
- Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer's disease. Lancet. 1997;349:1546–1549. - PubMed
-
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. Psych. Gerichtl. Med. 1907;64:146–148.
-
- Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Z. Gesamte Neurol. Psychiatr. 1911;4:356–385.
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

