EPO-mediated expansion of late-stage erythroid progenitors in the bone marrow initiates recovery from sublethal radiation stress
- PMID: 22889760
- PMCID: PMC3448262
- DOI: 10.1182/blood-2011-11-394304
EPO-mediated expansion of late-stage erythroid progenitors in the bone marrow initiates recovery from sublethal radiation stress
Abstract
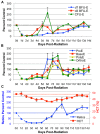
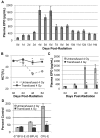
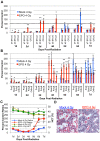
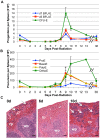
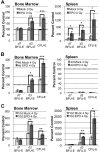
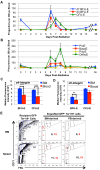
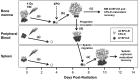
Erythropoiesis is a robust process of cellular expansion and maturation occurring in murine bone marrow and spleen. We previously determined that sublethal irradiation, unlike bleeding or hemolysis, depletes almost all marrow and splenic erythroblasts but leaves peripheral erythrocytes intact. To better understand the erythroid stress response, we analyzed progenitor, precursor, and peripheral blood compartments of mice post-4 Gy total body irradiation. Erythroid recovery initiates with rapid expansion of late-stage erythroid progenitors-day 3 burst-forming units and colony-forming units, associated with markedly increased plasma erythropoietin (EPO). Although initial expansion of late-stage erythroid progenitors is dependent on EPO, this cellular compartment becomes sharply down-regulated despite elevated EPO levels. Loss of EPO-responsive progenitors is associated temporally with a wave of maturing erythroid precursors in marrow and with emergence of circulating erythroid progenitors and subsequent reestablishment of splenic erythropoiesis. These circulating progenitors selectively engraft and mature in irradiated spleen after short-term transplantation, supporting the concept that bone marrow erythroid progenitors migrate to spleen. We conclude that sublethal radiation is a unique model of endogenous stress erythropoiesis, with specific injury to the extravascular erythron, expansion and maturation of EPO-responsive late-stage progenitors exclusively in marrow, and subsequent reseeding of extramedullary sites.
Figures
Similar articles
-
Functional Analysis of Erythroid Progenitors by Colony-Forming Assays.Methods Mol Biol. 2018;1698:117-132. doi: 10.1007/978-1-4939-7428-3_7. Methods Mol Biol. 2018. PMID: 29076087
-
Optimal erythroid cell production during erythropoietin treatment of mice occurs by exploiting the splenic microenvironment.Exp Hematol. 1993 Apr;21(4):496-501. Exp Hematol. 1993. PMID: 8462658
-
Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation.Blood. 2010 Dec 23;116(26):6072-81. doi: 10.1182/blood-2010-04-281840. Epub 2010 Sep 15. Blood. 2010. PMID: 20844235
-
End-stage renal disease following polycythemia vera: in vitro and in vivo response of erythroid progenitors to erythropoietin and effects of sera on normal erythropoiesis.Nephron. 1998;79(2):142-7. doi: 10.1159/000045016. Nephron. 1998. PMID: 9647492 Review.
-
Cellular mechanism of resistance to erythropoietin.Nephrol Dial Transplant. 1995;10 Suppl 6:27-30. doi: 10.1093/ndt/10.supp6.27. Nephrol Dial Transplant. 1995. PMID: 8524490 Review.
Cited by
-
The Hsp70 chaperone system: distinct roles in erythrocyte formation and maintenance.Haematologica. 2021 Jun 1;106(6):1519-1534. doi: 10.3324/haematol.2019.233056. Haematologica. 2021. PMID: 33832207 Free PMC article. Review.
-
Thioredoxin mitigates radiation-induced hematopoietic stem cell injury in mice.Stem Cell Res Ther. 2017 Nov 15;8(1):263. doi: 10.1186/s13287-017-0711-2. Stem Cell Res Ther. 2017. PMID: 29141658 Free PMC article.
-
Commonalities Between COVID-19 and Radiation Injury.Radiat Res. 2021 Jan 1;195(1):1-24. doi: 10.1667/RADE-20-00188.1. Radiat Res. 2021. PMID: 33064832 Free PMC article. Review.
-
Hemolysis-driven IFNα production impairs erythropoiesis by negatively regulating EPO signaling in sickle cell disease.Blood. 2024 Mar 14;143(11):1018-1031. doi: 10.1182/blood.2023021658. Blood. 2024. PMID: 38127913
-
FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis.Am J Hematol. 2014 Oct;89(10):954-63. doi: 10.1002/ajh.23786. Epub 2014 Jul 22. Am J Hematol. 2014. PMID: 24966026 Free PMC article.
References
-
- Morley A, Stohlman F., Jr Periodicity during recovery of erythropoiesis following irradiation. Blood. 1969;34(1):96–99. - PubMed
-
- Brady LW, Markoe AM, Ruggieri S, Brodsky I. The effect of sublethal x-irradiation on erythropoiesis in the mouse. Int J Radiat Oncol Biol Phys. 1976;1(5-6):471–479. - PubMed
-
- Okunewick JP, Phillips EL. Extended recovery lag in rat hematopoietic colony forming units following sublethal x-irradiation. J Lab Clin Med. 1972;79(4):550–558. - PubMed
-
- Ben-Ishay Z, Yoffey JM. Ultrastructural studies of erythroblastic islands of rat bone marrow. 3. Effects of sublethal irradiation. Lab Invest. 1974;30(3):320–332. - PubMed
-
- Nakeff A, McLellan WL, Bryan J, Valeriote FA. Response of Megakaryocyte, Erythroid, and Granulocyte-Macrophage Progenitor Cells in Mouse Bone Marrow to Gamma-Irradiation and Cyclophosphamide. In: Baum SJ, Ledney GD, editors. Exp Hematol Today 1979. New York: Springer-Verlag; 1979. pp. 99–104.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

