A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence
- PMID: 22889613
- PMCID: PMC3484205
- DOI: 10.1016/j.virol.2012.07.020
A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence
Abstract
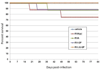
We are developing inactivated and live-attenuated rabies virus (RABV) vaccines expressing Ebola virus (EBOV) glycoprotein for use in humans and endangered wildlife, respectively. Here, we further characterize the pathogenesis of the live-attenuated RABV/EBOV vaccine candidates in mice in an effort to define their growth properties and potential for safety. RABV vaccines expressing GP (RV-GP) or a replication-deficient derivative with a deletion of the RABV G gene (RVΔG-GP) are both avirulent after intracerebral inoculation of adult mice. Furthermore, RVΔG-GP is completely avirulent upon intracerebral inoculation of suckling mice unlike parental RABV vaccine or RV-GP. Analysis of RVΔG-GP in the brain by quantitative PCR, determination of virus titer, and immunohistochemistry indicated greatly restricted virus replication. In summary, our findings indicate that RV-GP retains the attenuation phenotype of the live-attenuated RABV vaccine, and RVΔG-GP would appear to be an even safer alternative for use in wildlife or consideration for human use.
Published by Elsevier Inc.
Figures







Similar articles
-
Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses.J Virol. 2011 Oct;85(20):10605-16. doi: 10.1128/JVI.00558-11. Epub 2011 Aug 17. J Virol. 2011. PMID: 21849459 Free PMC article.
-
Controlled viral glycoprotein expression as a safety feature in a bivalent rabies-ebola vaccine.Virus Res. 2015 Feb 2;197:54-8. doi: 10.1016/j.virusres.2014.11.028. Epub 2014 Dec 4. Virus Res. 2015. PMID: 25481284 Free PMC article.
-
Genetically modified rabies virus-vectored Ebola virus disease vaccines are safe and induce efficacious immune responses in mice and dogs.Antiviral Res. 2017 Oct;146:36-44. doi: 10.1016/j.antiviral.2017.08.011. Epub 2017 Aug 16. Antiviral Res. 2017. PMID: 28822816
-
Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife.Zoonoses Public Health. 2009 Aug;56(6-7):262-9. doi: 10.1111/j.1863-2378.2008.01215.x. Zoonoses Public Health. 2009. PMID: 19486317 Review.
-
New approaches to the development of live attenuated rabies vaccines.Hybrid Hybridomics. 2002 Apr;21(2):129-34. doi: 10.1089/153685902317401735. Hybrid Hybridomics. 2002. PMID: 12031103 Review.
Cited by
-
Ebola vaccines in clinical trial: The promising candidates.Hum Vaccin Immunother. 2017 Jan 2;13(1):153-168. doi: 10.1080/21645515.2016.1225637. Epub 2016 Oct 20. Hum Vaccin Immunother. 2017. PMID: 27764560 Free PMC article. Review.
-
Rhabdovirus-based vaccine platforms against henipaviruses.J Virol. 2015 Jan;89(1):144-54. doi: 10.1128/JVI.02308-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320306 Free PMC article.
-
Replication-incompetent rabies virus vector harboring glycoprotein gene of lymphocytic choriomeningitis virus (LCMV) protects mice from LCMV challenge.PLoS Negl Trop Dis. 2018 Apr 16;12(4):e0006398. doi: 10.1371/journal.pntd.0006398. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29659579 Free PMC article.
-
Research Advances on the Interactions between Rabies Virus Structural Proteins and Host Target Cells: Accrued Knowledge from the Application of Reverse Genetics Systems.Viruses. 2021 Nov 16;13(11):2288. doi: 10.3390/v13112288. Viruses. 2021. PMID: 34835093 Free PMC article. Review.
-
Preclinical Development of Inactivated Rabies Virus-Based Polyvalent Vaccine Against Rabies and Filoviruses.J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S414-24. doi: 10.1093/infdis/jiv251. Epub 2015 Jun 10. J Infect Dis. 2015. PMID: 26063224 Free PMC article.
References
-
- Bermejo M, Rodriguez-Teijeiro JD, Illera G, Barroso A, Vila C, Walsh PD. Ebola outbreak killed 5000 gorillas. Science. 2006;314:1564. - PubMed
-
- Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, Holbrook MR, Freiberg AN, Bernbaum JG, Jahrling PB, Paragas J, Schnell MJ. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. Journal of virology. 2011;85:10605–10616. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

