Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification
- PMID: 22887744
- PMCID: PMC3562655
- DOI: 10.1002/jbmr.1733
Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification
Abstract
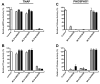
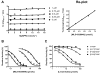
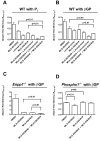
Medial vascular calcification (MVC) is common in patients with chronic kidney disease, obesity, and aging. MVC is an actively regulated process that resembles skeletal mineralization, resulting from chondro-osteogenic transformation of vascular smooth muscle cells (VSMCs). Here, we used mineralizing murine VSMCs to study the expression of PHOSPHO1, a phosphatase that participates in the first step of matrix vesicles-mediated initiation of mineralization during endochondral ossification. Wild-type (WT) VSMCs cultured under calcifying conditions exhibited increased Phospho1 gene expression and Phospho1(-/-) VSMCs failed to mineralize in vitro. Using natural PHOSPHO1 substrates, potent and specific inhibitors of PHOSPHO1 were identified via high-throughput screening and mechanistic analysis and two of these inhibitors, designated MLS-0390838 and MLS-0263839, were selected for further analysis. Their effectiveness in preventing VSMC calcification by targeting PHOSPHO1 function was assessed, alone and in combination with a potent tissue-nonspecific alkaline phosphatase (TNAP) inhibitor MLS-0038949. PHOSPHO1 inhibition by MLS-0263839 in mineralizing WT cells (cultured with added inorganic phosphate) reduced calcification in culture to 41.8% ± 2.0% of control. Combined inhibition of PHOSPHO1 by MLS-0263839 and TNAP by MLS-0038949 significantly reduced calcification to 20.9% ± 0.74% of control. Furthermore, the dual inhibition strategy affected the expression of several mineralization-related enzymes while increasing expression of the smooth muscle cell marker Acta2. We conclude that PHOSPHO1 plays a critical role in VSMC mineralization and that "phosphatase inhibition" may be a useful therapeutic strategy to reduce MVC.
Copyright © 2013 American Society for Bone and Mineral Research.
Conflict of interest statement
All the authors state that they have no conflicts of interest.
Figures




Similar articles
-
Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick.Bone. 2010 Apr;46(4):1146-55. doi: 10.1016/j.bone.2009.12.018. Epub 2010 Jan 4. Bone. 2010. PMID: 20053388 Free PMC article.
-
The use of tissue-nonspecific alkaline phosphatase (TNAP) and PHOSPHO1 inhibitors to affect mineralization by cultured cells.Methods Mol Biol. 2013;1053:125-34. doi: 10.1007/978-1-62703-562-0_8. Methods Mol Biol. 2013. PMID: 23860651
-
Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization.J Bone Miner Res. 2007 Apr;22(4):617-27. doi: 10.1359/jbmr.070108. J Bone Miner Res. 2007. PMID: 17227223
-
Role of bone-type tissue-nonspecific alkaline phosphatase and PHOSPO1 in vascular calcification.Curr Pharm Des. 2014;20(37):5821-8. doi: 10.2174/1381612820666140212193011. Curr Pharm Des. 2014. PMID: 24533943 Review.
-
The role of phosphatases in the initiation of skeletal mineralization.Calcif Tissue Int. 2013 Oct;93(4):299-306. doi: 10.1007/s00223-012-9672-8. Epub 2012 Nov 27. Calcif Tissue Int. 2013. PMID: 23183786 Free PMC article. Review.
Cited by
-
Biomolecules Orchestrating Cardiovascular Calcification.Biomolecules. 2021 Oct 7;11(10):1482. doi: 10.3390/biom11101482. Biomolecules. 2021. PMID: 34680115 Free PMC article. Review.
-
How To Build a Bone: PHOSPHO1, Biomineralization, and Beyond.JBMR Plus. 2019 Jul 7;3(7):e10202. doi: 10.1002/jbm4.10202. eCollection 2019 Jul. JBMR Plus. 2019. PMID: 31372594 Free PMC article. Review.
-
The Expression of PHOSPHO1, nSMase2 and TNAP is Coordinately Regulated by Continuous PTH Exposure in Mineralising Osteoblast Cultures.Calcif Tissue Int. 2016 Nov;99(5):510-524. doi: 10.1007/s00223-016-0176-9. Epub 2016 Jul 21. Calcif Tissue Int. 2016. PMID: 27444010 Free PMC article.
-
Targeted reduction of vascular Msx1 and Msx2 mitigates arteriosclerotic calcification and aortic stiffness in LDLR-deficient mice fed diabetogenic diets.Diabetes. 2014 Dec;63(12):4326-37. doi: 10.2337/db14-0326. Epub 2014 Jul 23. Diabetes. 2014. PMID: 25056439 Free PMC article.
-
Chronic Kidney Disease-Induced Arterial Media Calcification in Rats Prevented by Tissue Non-Specific Alkaline Phosphatase Substrate Supplementation Rather Than Inhibition of the Enzyme.Pharmaceutics. 2021 Jul 26;13(8):1138. doi: 10.3390/pharmaceutics13081138. Pharmaceutics. 2021. PMID: 34452102 Free PMC article.
References
-
- Chen NX, Moe SM. Arterial calcification in diabetes. Curr Diab Rep. 2003;3:28–32. - PubMed
-
- van der Zee S, Baber U, Elmariah S, Winston J, Fuster V. Cardiovascular risk factors in patients with chronic kidney disease. Nat Rev Cardiol. 2009;6:580–589. - PubMed
-
- Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

