Modulation of opioid receptor ligand affinity and efficacy using active and inactive state receptor models
- PMID: 22882801
- PMCID: PMC3465523
- DOI: 10.1111/cbdd.12014
Modulation of opioid receptor ligand affinity and efficacy using active and inactive state receptor models
Abstract
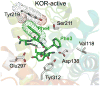
Mu opioid receptor (MOR) agonists are widely used for the treatment of pain; however, chronic use results in the development of tolerance and dependence. It has been demonstrated that coadministration of a MOR agonist with a delta opioid receptor (DOR) antagonist maintains the analgesia associated with MOR agonists, but with reduced negative side-effects. Using our newly refined opioid receptor models for structure-based ligand design, we have synthesized several pentapeptides with tailored affinity and efficacy profiles. In particular, we have obtained pentapeptides 8, Tyr-c(S-S)[DCys-1Nal-Nle-Cys]NH(2), and 12, Tyr-c(S-S)[DCys-1Nal-Nle-Cys]OH, which demonstrates high affinity and full agonist behavior at MOR, high affinity but very low efficacy for DOR, and minimal affinity for the kappa opioid receptor (KOR). Functional properties of these peptides as MOR agonists/DOR antagonists lacking undesired KOR activity make them promising candidates for future in vivo studies of MOR/DOR interactions. Subtle structural variation of 12, by substituting D-Cys(5) for L-Cys(5), generated analog 13, which maintains low nanomolar MOR and DOR affinity, but which displays no efficacy at either receptor. These results demonstrate the power and utility of accurate receptor models for structure-based ligand design, as well as the profound sensitivity of ligand function on its structure.
© 2012 John Wiley & Sons A/S.
Figures

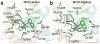
Similar articles
-
Pentapeptides displaying mu opioid receptor agonist and delta opioid receptor partial agonist/antagonist properties.J Med Chem. 2009 Dec 10;52(23):7724-31. doi: 10.1021/jm9007483. J Med Chem. 2009. PMID: 19788201 Free PMC article.
-
Further Optimization and Evaluation of Bioavailable, Mixed-Efficacy μ-Opioid Receptor (MOR) Agonists/δ-Opioid Receptor (DOR) Antagonists: Balancing MOR and DOR Affinities.J Med Chem. 2015 Nov 25;58(22):8952-69. doi: 10.1021/acs.jmedchem.5b01270. Epub 2015 Nov 13. J Med Chem. 2015. PMID: 26524472 Free PMC article.
-
Synthesis and evaluation of 4-substituted piperidines and piperazines as balanced affinity μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands.Bioorg Med Chem Lett. 2014 Jan 15;24(2):548-51. doi: 10.1016/j.bmcl.2013.12.021. Epub 2013 Dec 11. Bioorg Med Chem Lett. 2014. PMID: 24365161 Free PMC article.
-
Progress in the development of more effective and safer analgesics for pain management.Eur J Med Chem. 2019 Dec 1;183:111701. doi: 10.1016/j.ejmech.2019.111701. Epub 2019 Sep 16. Eur J Med Chem. 2019. PMID: 31550662 Review.
-
Bifunctional μ opioid and σ1 receptor ligands as novel analgesics with reduced side effects.Eur J Med Chem. 2021 Nov 5;223:113658. doi: 10.1016/j.ejmech.2021.113658. Epub 2021 Jun 18. Eur J Med Chem. 2021. PMID: 34175542 Review.
Cited by
-
Opioid peptidomimetics: leads for the design of bioavailable mixed efficacy μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands.J Med Chem. 2013 Mar 14;56(5):2139-49. doi: 10.1021/jm400050y. Epub 2013 Feb 27. J Med Chem. 2013. PMID: 23419026 Free PMC article.
-
Asymmetric synthesis and in vitro and in vivo activity of tetrahydroquinolines featuring a diverse set of polar substitutions at the 6 position as mixed-efficacy μ opioid receptor/δ opioid receptor ligands.ACS Chem Neurosci. 2015 Aug 19;6(8):1428-35. doi: 10.1021/acschemneuro.5b00100. Epub 2015 May 13. ACS Chem Neurosci. 2015. PMID: 25938166 Free PMC article.
-
Design, synthesis, and biological evaluation of a series of bifunctional ligands of opioids/SSRIs.Bioorg Med Chem. 2015 Mar 15;23(6):1251-9. doi: 10.1016/j.bmc.2015.01.047. Epub 2015 Feb 3. Bioorg Med Chem. 2015. PMID: 25703306 Free PMC article.
-
Translation of structure-activity relationships from cyclic mixed efficacy opioid peptides to linear analogues.Biopolymers. 2014 Jan;102(1):107-14. doi: 10.1002/bip.22437. Biopolymers. 2014. PMID: 24436042 Free PMC article.
-
Design synthesis and structure-activity relationship of 5-substituted (tetrahydronaphthalen-2yl)methyl with N-phenyl-N-(piperidin-2-yl)propionamide derivatives as opioid ligands.Bioorg Med Chem. 2016 Jan 15;24(2):85-91. doi: 10.1016/j.bmc.2015.11.030. Epub 2015 Nov 23. Bioorg Med Chem. 2016. PMID: 26712115 Free PMC article.
References
-
- Morphy R, Kay C, Rankovic Z. From Magic Bullets to Designed Multiple Ligands. Research Focus Reviews. 2004;9:641–52. - PubMed
-
- Morphy R, Rankovic Z. Designing Multiple Ligands - Medicinal Chemistry Strategies and Challenges. Current Pharmaceutical Design. 2009;15:587–600. - PubMed
-
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective Blockage of the Delta Opioid Receptors Prevents the Development of Morphine Tolerance and Dependence in Mice. The Journal of Pharmacology and Experimental Therapeutics. 1991;258:299–303. - PubMed
-
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska H, Coderre TJ. Attenuation of Morphine Tolerance and Dependence with the Highly Selective Delta Opioid Receptor Antagonist TIPP(psi) European Journal of Pharmacology. 1995;286:105–8. - PubMed
-
- Hepburn MJ, Little PJ, Gringas J, Khun CM. Differential Effects of Naltrindole on Morphine-Induced Tolerance and Physical Dependence in Rate. The Journal of Pharmacology and Experimental Therapeutics. 1997;281:1350–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

