Laninamivir octanoate and artificial surfactant combination therapy significantly increases survival of mice infected with lethal influenza H1N1 Virus
- PMID: 22879974
- PMCID: PMC3409853
- DOI: 10.1371/journal.pone.0042419
Laninamivir octanoate and artificial surfactant combination therapy significantly increases survival of mice infected with lethal influenza H1N1 Virus
Abstract
Background: Patients with influenza virus infection can develop severe pneumonia and acute respiratory distress syndrome (ARDS) which have a high mortality. Influenza virus infection is treated worldwide mainly by neuraminidase inhibitors (NAIs). However, monotherapy with NAIs is insufficient for severe pneumonia secondary to influenza virus infection. We previously demonstrated that mice infected with a lethal dose of influenza virus develop diffuse alveolar damage (DAD) with alveolar collapse similar to that seen in ARDS in humans. Additionally, pulmonary surfactant proteins were gradually increased in mouse serum, suggesting a decrease in pulmonary surfactant in the lung. Therefore, the present study examined whether combination therapy of NAI with exogenous artificial surfactant affects mortality of influenza virus-infected mice.
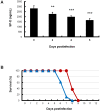
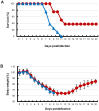
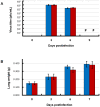
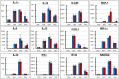
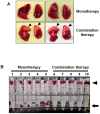
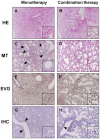
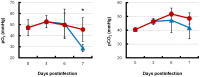
Methodology/principal findings: BALB/c mice were inoculated with several viral doses of influenza A/Puerto Rico/8/34 (PR8) virus (H1N1). The mice were additionally administered exogenous artificial surfactant in the presence or absence of a new NAI, laninamivir octanoate. Mouse survival, body weight and general condition were observed for up to 20 days after inoculation. Viral titer and cytokine/chemokine levels in the lungs, lung weight, pathological analysis, and blood O(2) and CO(2) pressures were evaluated. Infected mice treated with combination therapy of laninamivir octanoate with artificial surfactant showed a significantly higher survival rate compared with those that received laninamivir octanoate monotherapy (p = 0.003). However, virus titer, lung weight and cytokine/chemokine responses were not different between the groups. Histopathological examination, a hydrostatic lung test and blood gas analysis showed positive results in the combination therapy group.
Conclusions/significance: Combination therapy of laninamivir octanoate with artificial surfactant reduces lethality in mice infected with influenza virus, and eventually suppresses DAD formation and preserves lung function. This combination could be effective for prevention of severe pneumonia secondary to influenza virus infection in humans, which is not improved by NAI monotherapy.
Conflict of interest statement
Figures
Similar articles
-
In Vitro and In Vivo Characterization of Novel Neuraminidase Substitutions in Influenza A(H1N1)pdm09 Virus Identified Using Laninamivir-Mediated In Vitro Selection.J Virol. 2019 Mar 5;93(6):e01825-18. doi: 10.1128/JVI.01825-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30602610 Free PMC article.
-
Serial histopathological examination of the lungs of mice infected with influenza A virus PR8 strain.PLoS One. 2011;6(6):e21207. doi: 10.1371/journal.pone.0021207. Epub 2011 Jun 20. PLoS One. 2011. PMID: 21701593 Free PMC article.
-
Characterization of drug-resistant influenza virus A(H1N1) and A(H3N2) variants selected in vitro with laninamivir.Antimicrob Agents Chemother. 2014 Sep;58(9):5220-8. doi: 10.1128/AAC.03313-14. Epub 2014 Jun 23. Antimicrob Agents Chemother. 2014. PMID: 24957832 Free PMC article.
-
Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza.Expert Rev Anti Infect Ther. 2011 Oct;9(10):851-7. doi: 10.1586/eri.11.112. Expert Rev Anti Infect Ther. 2011. PMID: 21973296 Review.
-
Neuraminidase inhibitors for preventing and treating influenza in children.Cochrane Database Syst Rev. 2012 Jan 18;1:CD002744. doi: 10.1002/14651858.CD002744.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2012 Apr 18;(4):CD002744. doi: 10.1002/14651858.CD002744.pub4 PMID: 22258949 Updated. Review.
Cited by
-
Surfactants - Compounds for inactivation of SARS-CoV-2 and other enveloped viruses.Curr Opin Colloid Interface Sci. 2021 Oct;55:101479. doi: 10.1016/j.cocis.2021.101479. Epub 2021 Jun 12. Curr Opin Colloid Interface Sci. 2021. PMID: 34149296 Free PMC article. Review.
-
A Lipopolysaccharide from Pantoea Agglomerans Is a Promising Adjuvant for Sublingual Vaccines to Induce Systemic and Mucosal Immune Responses in Mice via TLR4 Pathway.PLoS One. 2015 May 15;10(5):e0126849. doi: 10.1371/journal.pone.0126849. eCollection 2015. PLoS One. 2015. PMID: 25978818 Free PMC article.
-
Effect of H2 treatment in a mouse model of rheumatoid arthritis-associated interstitial lung disease.J Cell Mol Med. 2019 Oct;23(10):7043-7053. doi: 10.1111/jcmm.14603. Epub 2019 Aug 19. J Cell Mol Med. 2019. PMID: 31424157 Free PMC article.
-
Mechanistic Understanding of Lung Inflammation: Recent Advances and Emerging Techniques.J Inflamm Res. 2022 Jun 15;15:3501-3546. doi: 10.2147/JIR.S282695. eCollection 2022. J Inflamm Res. 2022. PMID: 35734098 Free PMC article. Review.
-
Antiviral combinations for severe influenza.Lancet Infect Dis. 2014 Dec;14(12):1259-70. doi: 10.1016/S1473-3099(14)70821-7. Epub 2014 Sep 8. Lancet Infect Dis. 2014. PMID: 25213733 Free PMC article. Review.
References
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team (2009) Dawood FS, Jain S, Finelli L, Shaw MW, et al. (2009) Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360 25 2605–2615. - PubMed
-
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, et al. (2009) Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in mexico. N Engl J Med 361 7 680–689. - PubMed
-
- Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, et al. (2004) Avian influenza A (H5N1) in 10 patients in vietnam. N Engl J Med 350 12 1179–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

