Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes
- PMID: 22879928
- PMCID: PMC3411774
- DOI: 10.1371/journal.pone.0042277
Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes
Abstract
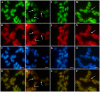
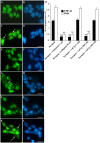
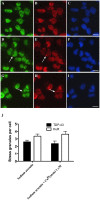
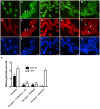
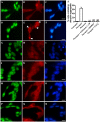
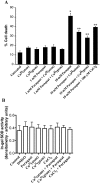
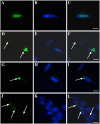
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal, motor neuron disease with no effective long-term treatment options. Recently, TDP-43 has been identified as a key protein in the pathogenesis of some cases of ALS. Although the role of TDP-43 in motor neuron degeneration is not yet known, TDP-43 has been shown to accumulate in RNA stress granules (SGs) in cell models and in spinal cord tissue from ALS patients. The SG association may be an early pathological change to TDP-43 metabolism and as such a potential target for therapeutic intervention. Accumulation of TDP-43 in SGs induced by inhibition of mitochondrial activity can be inhibited by modulation of cellular kinase activity. We have also found that treatment of cells and animal models of neurodegeneration, including an ALS model, with bioavailable bis(thiosemicarbazonato)copper(II) complexes (Cu(II)(btsc)s) can modulate kinase activity and induce neuroprotective effects. In this study we examined the effect of diacetylbis(-methylthiosemicarbazonato)copper(II) (Cu(II)(atsm)) and glyoxalbis(-methylthiosemicarbazonato)copper(II) (Cu(II)(gtsm)) on TDP-43-positive SGs induced in SH-SY5Y cells in culture. We found that the Cu(II)(btsc)s blocked formation of TDP-43-and human antigen R (HuR)-positive SGs induced by paraquat. The Cu(II)(btsc)s protected neurons from paraquat-mediated cell death. These effects were associated with inhibition of ERK phosphorylation. Co-treatment of cultures with either Cu(II)(atsm) or an ERK inhibitor, PD98059 both prevented ERK activation and blocked formation of TDP-43-and HuR-positive SGs. Cu(II)(atsm) treatment or ERK inhibition also prevented abnormal ubiquitin accumulation in paraquat-treated cells suggesting a link between prolonged ERK activation and abnormal ubiquitin metabolism in paraquat stress and inhibition by Cu. Moreover, Cu(II)(atsm) reduced accumulation of C-terminal (219-414) TDP-43 in transfected SH-SY5Y cells. These results demonstrate that Cu(II)(btsc) complexes could potentially be developed as a neuroprotective agent to modulate neuronal kinase function and inhibit TDP-43 aggregation. Further studies in TDP-43 animal models are warranted.
Conflict of interest statement
Figures

Similar articles
-
Endogenous TDP-43 localized to stress granules can subsequently form protein aggregates.Neurochem Int. 2012 Mar;60(4):415-24. doi: 10.1016/j.neuint.2012.01.019. Epub 2012 Jan 27. Neurochem Int. 2012. PMID: 22306778
-
Sustained activation of glial cell epidermal growth factor receptor by bis(thiosemicarbazonato) metal complexes is associated with inhibition of protein tyrosine phosphatase activity.J Med Chem. 2009 Nov 12;52(21):6606-20. doi: 10.1021/jm9007938. J Med Chem. 2009. PMID: 19807095
-
Mechanisms controlling the cellular accumulation of copper bis(thiosemicarbazonato) complexes.Inorg Chem. 2011 Oct 3;50(19):9594-605. doi: 10.1021/ic201334q. Epub 2011 Sep 1. Inorg Chem. 2011. PMID: 21882803
-
Stress granules at the intersection of autophagy and ALS.Brain Res. 2016 Oct 15;1649(Pt B):189-200. doi: 10.1016/j.brainres.2016.05.022. Epub 2016 May 13. Brain Res. 2016. PMID: 27181519 Free PMC article. Review.
-
Stress granules in neurodegeneration--lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma.FEBS J. 2013 Sep;280(18):4348-70. doi: 10.1111/febs.12287. Epub 2013 May 9. FEBS J. 2013. PMID: 23587065 Review.
Cited by
-
Targeting stress granules: A novel therapeutic strategy for human diseases.Pharmacol Res. 2020 Nov;161:105143. doi: 10.1016/j.phrs.2020.105143. Epub 2020 Aug 16. Pharmacol Res. 2020. PMID: 32814168 Free PMC article. Review.
-
Emerging Therapies and Novel Targets for TDP-43 Proteinopathy in ALS/FTD.Neurotherapeutics. 2022 Jul;19(4):1061-1084. doi: 10.1007/s13311-022-01260-5. Epub 2022 Jul 5. Neurotherapeutics. 2022. PMID: 35790708 Free PMC article. Review.
-
Copper-ATSM as a Treatment for ALS: Support from Mutant SOD1 Models and Beyond.Life (Basel). 2020 Nov 4;10(11):271. doi: 10.3390/life10110271. Life (Basel). 2020. PMID: 33158182 Free PMC article. Review.
-
Copper toxicity and deficiency: the vicious cycle at the core of protein aggregation in ALS.Front Mol Neurosci. 2024 Jul 9;17:1408159. doi: 10.3389/fnmol.2024.1408159. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39050823 Free PMC article. Review.
-
Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder.Acta Neuropathol Commun. 2014 Feb 28;2:25. doi: 10.1186/2051-5960-2-25. Acta Neuropathol Commun. 2014. PMID: 24581221 Free PMC article.
References
-
- King AE, Dickson TC, Blizzard CA, Woodhouse A, Foster SS, et al. (2011) Neuron-glia interactions underlie ALS-like axonal cytoskeletal pathology. Neurobiol Aging 32: 459–69. - PubMed
-
- Bowling AC, Schulz JB, Brown RHJ, Beal MF (1993) Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem 61: 2322–2325. - PubMed
-
- Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364: 362. - PubMed
-
- Barber SC, Shaw PJ (2010) Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med 48: 629–641. - PubMed
-
- Swarup V, Julien JP (2010) ALS pathogenesis: Recent insights from genetics and mouse models. Prog Neuropsychopharmacol Biol Psychiatry 35: 363–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

