Effects of Perinuclear Chromosome Tethers in the Telomeric URA3/5FOA System Reflect Changes to Gene Silencing and not Nucleotide Metabolism
- PMID: 22876257
- PMCID: PMC3410493
- DOI: 10.3389/fgene.2012.00144
Effects of Perinuclear Chromosome Tethers in the Telomeric URA3/5FOA System Reflect Changes to Gene Silencing and not Nucleotide Metabolism
Abstract
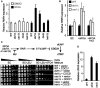
Telomeres are repetitive DNA sequences that protect the ends of linear chromosomes. Telomeres also recruit histone deacetylase complexes that can then spread along chromosome arms and repress the expression of subtelomeric genes in a process known as telomere position effect (TPE). In the budding yeast Saccharomyces cerevisiae, association of telomeres with the nuclear envelope is thought to promote TPE by increasing the local concentration of histone deacetylase complexes at chromosome ends. Importantly, our understanding of TPE stems primarily from studies that employed marker genes inserted within yeast subtelomeres. In particular, the prototrophic marker URA3 is commonly used to assay TPE by negative selection on media supplemented with 5-fluoro-orotic acid (5FOA). Recent findings suggested that decreased growth on 5FOA-containing media may not always indicate increased expression of a telomeric URA3 reporter, but can rather reflect an increase in ribonucleotide reductase (RNR) function and nucleotide metabolism. Thus, we set out to test if the 5FOA sensitivity of subtelomeric URA3-harboring cells in which we deleted various factors implicated in perinuclear telomere tethering reflects changes to TPE and/or RNR. We report that RNR inhibition restores 5FOA resistance to cells lacking RNR regulatory factors but not any of the major telomere tethering and silencing factors, including Sir2, cohibin, Mps3, Heh1, and Esc1. In addition, we find that the disruption of tethering pathways in which these factors participate increases the level of URA3 transcripts originating from the telomeric reporter gene and abrogates silencing of subtelomeric HIS3 reporter genes without altering RNR gene expression. Thus, increased 5FOA sensitivity of telomeric URA3-harboring cells deficient in telomere tethers reflects the dysregulation of TPE but not RNR. This is key to understanding relationships between telomere positioning, chromatin silencing, and lifespan.
Keywords: Esc1; Heh1; Mps3; SIR; URA3/5FOA; cohibin; ribonucleotide reductase; telomere position effect.
Figures




Similar articles
-
A common telomeric gene silencing assay is affected by nucleotide metabolism.Mol Cell. 2011 Apr 8;42(1):127-36. doi: 10.1016/j.molcel.2011.03.007. Mol Cell. 2011. PMID: 21474074 Free PMC article.
-
Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains.Dev Cell. 2011 Jun 14;20(6):867-79. doi: 10.1016/j.devcel.2011.05.014. Dev Cell. 2011. PMID: 21664583
-
Subtelomeric elements influence but do not determine silencing levels at Saccharomyces cerevisiae telomeres.Genetics. 2007 Dec;177(4):2541-6. doi: 10.1534/genetics.107.079806. Genetics. 2007. PMID: 18073447 Free PMC article.
-
Transcriptional silencing at Saccharomyces telomeres: implications for other organisms.Oncogene. 2002 Jan 21;21(4):512-21. doi: 10.1038/sj.onc.1205078. Oncogene. 2002. PMID: 11850776 Review.
-
Rnr1's role in telomere elongation cannot be replaced by Rnr3: a role beyond dNTPs?Curr Genet. 2018 Jun;64(3):547-550. doi: 10.1007/s00294-017-0779-3. Epub 2017 Nov 8. Curr Genet. 2018. PMID: 29119271 Review.
Cited by
-
Defining the Damaged DNA Mobility Paradox as Revealed by the Study of Telomeres, DSBs, Microtubules and Motors.Front Genet. 2018 Mar 20;9:95. doi: 10.3389/fgene.2018.00095. eCollection 2018. Front Genet. 2018. PMID: 29616083 Free PMC article.
-
Nucleolar Pol II interactome reveals TBPL1, PAF1, and Pol I at intergenic rDNA drive rRNA biogenesis.Nat Commun. 2024 Nov 6;15(1):9603. doi: 10.1038/s41467-024-54002-w. Nat Commun. 2024. PMID: 39505901 Free PMC article.
-
Analysis of epigenetic stability and conversions in Saccharomyces cerevisiae reveals a novel role of CAF-I in position-effect variegation.Nucleic Acids Res. 2013 Oct;41(18):8475-88. doi: 10.1093/nar/gkt623. Epub 2013 Jul 17. Nucleic Acids Res. 2013. PMID: 23863839 Free PMC article.
-
Breaking an epigenetic chromatin switch: curious features of hysteresis in Saccharomyces cerevisiae telomeric silencing.PLoS One. 2014 Dec 23;9(12):e113516. doi: 10.1371/journal.pone.0113516. eCollection 2014. PLoS One. 2014. PMID: 25536038 Free PMC article.
References
-
- Aparicio O. M., Billington B. L., Gottschling D. E. (1991). Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66, 1279–1287 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

