CCR5 antagonism impacts vaccination response and immune profile in HIV-1 infection
- PMID: 22875102
- PMCID: PMC3510292
- DOI: 10.2119/molmed.2012.00206
CCR5 antagonism impacts vaccination response and immune profile in HIV-1 infection
Abstract
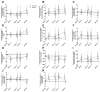
Maraviroc (MVC) is the first licensed antiretroviral therapeutic agent to target a host cell surface molecule, and successful HIV-1 entry blockade by this C-C chemokine receptor type 5 (CCR5)-antagonist potentiates immunomodulation. We hypothesized that MVC intensification impacts immunization responses, T-cell phenotype, function and delayed type hypersensitivity (DTH) in HIV-1(+) subjects. A 24-wk, double-blinded, placebo-controlled study of the addition of MVC to suppressive antiretroviral therapy in HIV-1(+) persons was performed. Subjects received DTH tests, intramuscular tetanus, meningococcal and oral cholera immunizations. Antibody titers, T-cell function and phenotype were assessed. Of 157 patients referred, 47 were randomized 1:1; MVC:placebo. MVC enhanced meningococcal neo-immunization, blunted cholera response and expedited lymphoproliferation to tetanus boost, without affecting recall humoral response. Anti-HIV-1 group-specific antigen (Gag) and tetanus toxoid (TTox) function improved significantly, HIV-1-associated CD8 T-cell skewing normalized, and the percentage of late-stage and major histocompatibility complex (MHC) class II expressing CD4 T-cells increased. Activated CD4(+) CD38(+) human leukocyte antigen (HLA)-DR(+) T-cells declined, and costimulation shifted to coinhibition. DTH was unchanged. Maraviroc intensification, through antagonism of the cell surface molecule CCR5, favorably influences immune profiles of HIV-1(+) patients, supporting its immunomodulatory use in HIV-1 infection and potentially in other immunologically relevant settings.
Figures

Similar articles
-
Maraviroc Intensification of cART in Patients with Suboptimal Immunological Recovery: A 48-Week, Placebo-Controlled Randomized Trial.PLoS One. 2015 Jul 24;10(7):e0132430. doi: 10.1371/journal.pone.0132430. eCollection 2015. PLoS One. 2015. PMID: 26208341 Free PMC article. Clinical Trial.
-
Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected.PLoS One. 2011;6(12):e27864. doi: 10.1371/journal.pone.0027864. Epub 2011 Dec 8. PLoS One. 2011. PMID: 22174752 Free PMC article. Clinical Trial.
-
The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial.Blood. 2013 Jun 6;121(23):4635-46. doi: 10.1182/blood-2012-06-436345. Epub 2013 Apr 15. Blood. 2013. PMID: 23589670 Free PMC article. Clinical Trial.
-
Clinical utility of maraviroc.Clin Drug Investig. 2011;31(8):527-542. doi: 10.2165/11590700-000000000-00000. Clin Drug Investig. 2011. PMID: 21595497 Review.
-
Maraviroc: a review of its use in HIV infection and beyond.Drug Des Devel Ther. 2015 Oct 1;9:5447-68. doi: 10.2147/DDDT.S90580. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26491256 Free PMC article. Review.
Cited by
-
Multifarious immunotherapeutic approaches to cure HIV-1 infection.Hum Vaccin Immunother. 2015;11(9):2287-93. doi: 10.1080/21645515.2015.1021523. Hum Vaccin Immunother. 2015. PMID: 26048144 Free PMC article. Clinical Trial.
-
Effect of maraviroc intensification on HIV-1-specific T cell immunity in recently HIV-1-infected individuals.PLoS One. 2014 Jan 27;9(1):e87334. doi: 10.1371/journal.pone.0087334. eCollection 2014. PLoS One. 2014. PMID: 24475275 Free PMC article. Clinical Trial.
-
Association between a Suppressive Combined Antiretroviral Therapy Containing Maraviroc and the Hepatitis B Virus Vaccine Response.Antimicrob Agents Chemother. 2017 Dec 21;62(1):e02050-17. doi: 10.1128/AAC.02050-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29084751 Free PMC article.
-
Maraviroc Intensification of cART in Patients with Suboptimal Immunological Recovery: A 48-Week, Placebo-Controlled Randomized Trial.PLoS One. 2015 Jul 24;10(7):e0132430. doi: 10.1371/journal.pone.0132430. eCollection 2015. PLoS One. 2015. PMID: 26208341 Free PMC article. Clinical Trial.
-
Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines.Vaccines (Basel). 2022 Nov 11;10(11):1906. doi: 10.3390/vaccines10111906. Vaccines (Basel). 2022. PMID: 36423002 Free PMC article. Review.
References
-
- Samson M, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

