Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia
- PMID: 22870988
- PMCID: PMC3580576
- DOI: 10.1186/ar4011
Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia
Abstract
Introduction: Inflammatory arthritis is a progressive disease with chronic inflammation of joints, which is mainly characterized by the infiltration of immune cells and synovial hyperproliferation. Monocytes migrate towards inflamed areas and differentiate into macrophages. In inflamed tissues, much lower oxygen levels (hypoxia) are present in comparison to the peripheral blood. Hence, a metabolic adaptation process must take place. Other studies suggest that Hypoxia Inducible Factor 1-alpha (HIF-1α) may regulate this process, but the mechanism involved for human monocytes is not yet clear. To address this issue, we analyzed the expression and function of HIF-1α in monocytes and macrophages, but also considered alternative pathways involving nuclear factor of kappa light polypeptide gene enhancer in B-cells (NFκB).
Methods: Isolated human CD14⁺ monocytes were incubated under normoxia and hypoxia conditions with or without phorbol 12-myristate 13-acetate (PMA) stimulation, respectively. Nuclear and cytosolic fractions were prepared in order to detect HIF-1α and NFκB by immunoblot. For the experiments with macrophages, primary human monocytes were differentiated into human monocyte derived macrophages (hMDM) using human macrophage colony-stimulating factor (hM-CSF). The effects of normoxia and hypoxia on gene expression were compared between monocytes and hMDMs using quantitative PCR (quantitative polymerase chain reaction).
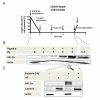
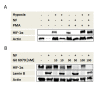
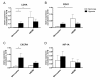
Results: We demonstrate, using primary human monocytes and hMDM, that the localization of transcription factor HIF-1α during the differentiation process is shifted from the cytosol (in monocytes) into the nucleus (in macrophages), apparently as an adaptation to a low oxygen environment. For this localization change, protein kinase C alpha/beta 1 (PKC-α/β₁) plays an important role. In monocytes, it is NFκB1, and not HIF-1α, which is of central importance for the expression of hypoxia-adjusted genes.
Conclusions: These data demonstrate that during differentiation of monocytes into macrophages, crucial cellular adaptation mechanisms are decisively changed.
Figures



Similar articles
-
Regulation of the Hif-system by micro-RNA 17 and 20a - role during monocyte-to-macrophage differentiation.Mol Immunol. 2013 Dec;56(4):442-51. doi: 10.1016/j.molimm.2013.06.014. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911400
-
Normoxic stabilization of hypoxia-inducible factor-1alpha by modulation of the labile iron pool in differentiating U937 macrophages: effect of natural resistance-associated macrophage protein 1.Cancer Res. 2006 Mar 1;66(5):2600-7. doi: 10.1158/0008-5472.CAN-05-2351. Cancer Res. 2006. PMID: 16510578
-
NRF2 Signaling Negatively Regulates Phorbol-12-Myristate-13-Acetate (PMA)-Induced Differentiation of Human Monocytic U937 Cells into Pro-Inflammatory Macrophages.PLoS One. 2015 Jul 29;10(7):e0134235. doi: 10.1371/journal.pone.0134235. eCollection 2015. PLoS One. 2015. PMID: 26222138 Free PMC article.
-
Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration.Immunobiology. 2008;213(9-10):733-49. doi: 10.1016/j.imbio.2008.07.031. Epub 2008 Sep 21. Immunobiology. 2008. PMID: 18926289 Review.
-
Hypoxia inducible factor-1α is an important regulator of macrophage biology.Heliyon. 2023 Jun 9;9(6):e17167. doi: 10.1016/j.heliyon.2023.e17167. eCollection 2023 Jun. Heliyon. 2023. PMID: 37484306 Free PMC article. Review.
Cited by
-
Macrophage phenotype in response to ECM bioscaffolds.Semin Immunol. 2017 Feb;29:2-13. doi: 10.1016/j.smim.2017.04.004. Epub 2017 Jul 21. Semin Immunol. 2017. PMID: 28736160 Free PMC article. Review.
-
Method to Regulate Monocyte Function by Silencing HIF-1α mRNA in a Model of Retinal Neovascularization.ACS Appl Nano Mater. 2023 Dec 6;6(24):22939-22946. doi: 10.1021/acsanm.3c04300. eCollection 2023 Dec 22. ACS Appl Nano Mater. 2023. PMID: 38148985 Free PMC article.
-
Bioenergetic analysis of human peripheral blood mononuclear cells.Clin Exp Immunol. 2015 Oct;182(1):69-80. doi: 10.1111/cei.12662. Epub 2015 Jul 24. Clin Exp Immunol. 2015. PMID: 26032049 Free PMC article.
-
Hypoxia primes human normal prostate epithelial cells and cancer cell lines for the NLRP3 and AIM2 inflammasome activation.Oncotarget. 2016 May 10;7(19):28183-94. doi: 10.18632/oncotarget.8594. Oncotarget. 2016. PMID: 27058421 Free PMC article.
-
Mitochondrial mechanisms in the pathogenesis of chronic inflammatory musculoskeletal disorders.Cell Biosci. 2024 Jun 8;14(1):76. doi: 10.1186/s13578-024-01259-9. Cell Biosci. 2024. PMID: 38849951 Free PMC article. Review.
References
-
- Biniecka M, Kennedy A, Ng CT, Chang TC, Balogh E, Fox E, Veale DJ, Fearon U, O'Sullivan JN. Successful tumour necrosis factor (TNF) blocking therapy suppresses oxidative stress and hypoxia-induced mitochondrial mutagenesis in inflammatory arthritis. Arthritis Res Ther. 2011;13:R121. doi: 10.1186/ar3424. - DOI - PMC - PubMed
-
- Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today. 2000;21:192–199. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

